Disputes regarding the correct charging of a calcium battery have been going on for a long time. One half of car owners advocates a voltage of 14.8 V (± 0.4 V) when charging, while the other claims that for a 100% charge of a calcium battery, a voltage of at least 16.0 V is required. At the same time, each side gives its own “ convincing” arguments to prove the infallibility of his theory. Let's try to figure out together what is the reason for such heated debates and is there the only correct way to charge a calcium battery?
For reference: the maximum soluble value of calcium (Ca) in lead (Pb) is 0.1% of the bulk. The lead plates of modern batteries contain from 0.06% to 0.09% calcium. This ratio was determined experimentally and is the most optimal.
Further reading will be useful in understanding why today traditional batteries with lead-antimony alloy grids are a thing of the past, and they have been replaced by batteries with lead-calcium alloy grids. Understanding these simple truths will help us see “from which side,” figuratively speaking, we need to approach charging calcium batteries.
No one wants the benefits of calcium batteries
Did you know that the first calcium battery was created in 1932 by an American company? At that time, this was the biggest progress in the lead-acid battery market, because these batteries acquired a number of advantages over traditional antimony batteries. They had much lower self-discharge (retained accumulated energy for a long time) and very low water consumption (they practically did not “boil” during operation), which made them the undisputed leaders in unpretentious maintenance.
It would seem that calcium batteries are the future! But no. In practice, “calcium” technology has not been widely used until now. Why? Because along with the advantages, calcium batteries also have significant disadvantages. Among them:
- It is difficult to obtain castings with a constant calcium content due to its rapid burnout during the melting process. This process was labor-intensive and financially expensive, which greatly hampered the spread of “calcium technology.”
- Significant loss of capacity of positive electrodes during deep discharges as a result of:
- intergranular corrosion of a lead-calcium alloy (Pb-Ca), which makes the positive electrode brittle and brittle, worsening its conductive properties;
- deterioration of contact between the current-carrying grid and the active mass due to the formation between them of a dense layer of lead sulfate (PbSO4), which “does not dissolve” (does not oxidize in PbO2) even when fully charged (apparently, it plays an important role in the formation of this layer calcium sulfate CaSO4);
- weak adhesion of the corrosion film to both the active mass and the current conductor, which increases the tendency of the active mass to melt.
- The difficulty of controlling the degree of charge based on the density of the electrolyte due to its stratification or stratification. Unlike traditional antimony batteries, calcium batteries do not “boil” and the electrolyte does not mix during charging, which means that acid, which is heavier than water, accumulates at the bottom of the cans, displacing less dense layers of electrolyte upward.
- The capacity of a battery with grids made of a lead-calcium alloy operating in a constant charge mode was lower than the capacity of a similar battery with grids made of a lead-antimony alloy.
Information obtained from sources: “Modern theory of a lead-acid battery”, authors: M.A. Dasoyan, I.A. Aguf, 1975, pp. 183-185. “Lead batteries”, authors: A.I. Rusin, L.D. Khegay, 2009, pp. 54-55.
The above disadvantages have significantly narrowed the scope of application of calcium batteries. They were used only in cases where the ability of the battery to maintain a charge over time was of paramount importance. In other words, the battery had to hold a charge for a long time. In most other cases, traditional antimony batteries were preferred.
Disadvantages of calcium batteries.
Unfortunately, there are no ideal things in our lives. Therefore, calcium batteries also have a number of disadvantages.
- Sensitivity to deep discharges. This is the main difference between calcium batteries and their hybrid and antimony counterparts. It is highly not recommended to discharge calcium batteries below a voltage of 12 V. With just one deep discharge, such a battery will lose a fifth of its capacity. With a single full discharge, the battery loses half of its capacity, while a device that has survived 9-10 discharges becomes completely unusable.
- Quite a high cost. This is due to the expensive and complex production process.
- Not suitable for urban travel mode. Long downtime, if the car is used infrequently and for short distances, has a negative and even detrimental effect on calcium batteries.
Please note that calcium batteries are only suitable for use in automobiles. We advise you to refrain from installing such devices in a boat or boat (where they may be subject to deep discharge).
Demand creates supply
Why are calcium batteries used everywhere now? Maybe science has found a way to get rid of all the above disadvantages? Not really. The main factor here is the basic law of a market economy, which can be formulated as follows: “Demand creates supply.”
Automobile boom
Remember how many cars there were in the yards of your houses just 30 years ago? How many are there now? In any city, courtyards are literally filled with cars. There is a car boom. Previously, a car was a luxury and literally “the specks of dust were blown off”, but now? Do you think that owners of cars that “live” on the street do not sleep at night, thinking about how to service the battery on their car? Nothing like this.
Set it and forget it
People's priorities have changed a lot. The car has become a common means of transportation, and the choice of battery has shifted towards the increasingly popular “set it and forget it” principle. Whatever they say, most motorists don’t care what happens to their battery. They just don't want to deal with their service.
Here are the words of one motorist: “As practice has shown, all 3 new cars that I bought (over the last 15 years) had batteries that lasted 2 years, maximum 3” (I’ll add that this motorist is from the northern regions of Russia). Then I asked him: “Have you tried charging it?” To which he replied: “Why? It’s easier to buy a new one.” How do you like this answer? Strange, isn't it? However, there are more and more such people every day.
Competition between battery manufacturers
Now it’s clear why battery manufacturers go out of their way to offer consumers a battery that doesn’t require any attention, in a word – “set it and forget it.” And the competition among battery manufacturers is now being conducted in one single area - whose battery will last longer without any maintenance
.
Ca/Ag
There is another, less common type of calcium battery - silver-calcium battery (Ca/Ag). It’s easy to guess that, by analogy with the previous example, they are simply called silver in everyday life. The plates of such batteries are made of a lead-calcium alloy, to which very little silver is added. The presence of this element practically eliminates the battery from the disadvantages of Ca/Ca type models, while retaining all their advantages. The only problem with such batteries is the high price, which is due not only to the cost of raw materials, but also to the complexity of manufacturing the battery.
Calcium – green light or “return of the prodigal parrot”
How conveniently the forgotten “calcium” technology turned out to be, which makes it possible to produce “maintenance-free batteries”. Yes, it was the doping of lead with calcium that made it possible to increase the hydrogen overvoltage or, to put it simply, the battery began to “boil” at a higher voltage (16.0 V). When the car's on-board voltage is 14.4 V, calcium batteries do not “boil” at all and do not require constant topping up with distilled water, which means they require only minimal maintenance. A calcium battery has another bonus - its ability to hold a charge for a long time. Let's say, if your car sits in the parking lot for a couple of months, then when you return for it, you will have every chance of successfully starting the engine. However, this is where its advantages end.
And nothing has changed …
But what about the shortcomings, you ask?..... No way! Of course, engineers are trying to “extend the life” of calcium batteries by experimenting with “cooking recipes” for conductive grids, as well as the coating itself, from which the active mass is subsequently formed. Plus, various “improvers” are added to the electrolyte. Even the production cost of the lead-calcium alloy was reduced (due to improved technology), but in a global sense, problems with calcium were and remain.
However, this is no longer important. It is important that now the consumer can be offered a product that meets his MAIN criterion: “set it and forget it.”
Official instructions
How do manufacturers recommend charging a calcium battery? They usually attach short instructions to it, in which they write, without any clear explanation, what to do. One of these official instructions is now on the table, and we will take key points from it.
So, it says the following:
- A calcium battery should be charged with a current equal to 10% of its capacity.
- When the charge voltage reaches 14.4 V, the current must be halved and charged for 10 hours.
- Upon completion of the charge, it is necessary to check the density of the electrolyte and, in case of high concentration, adjust it by adding water.
- Each time after adjusting the density, the battery must be charged at a voltage of 16 V, and charged for 40 minutes.
This instruction is very good. But it has several shortcomings. The author of this instruction did not say a word about those cases when the density at the end of the charge, on the contrary, is low. He also didn’t say anything about the fact that 16 V could have an adverse effect on the car’s electronics if it occurred to you to charge the battery without disconnecting it from the on-board network. In addition, there is nothing in this instruction for those whose chargers can only be adjusted by voltage, or are generally automatic.
Therefore, let’s add a little to this instruction. Or rather, we’ll rewrite it from scratch, not forgetting about non-standard situations. We will also add a few recommendations to it that will extend the life of the calcium battery.
or the basis of a marketing strategy
The situation today is such that, under actual operating conditions of a calcium battery, charging at 14.8 V provokes deep sulfation of the plates, and charging at 16.2 V contributes to faster melting of the active mass. In both cases, the battery capacity decreases. Each of these SEPARATE charging methods is detrimental to a calcium battery, no matter how you look at it.
However, the biggest evil that a calcium battery conceals is its intolerance to deep cycling. To put it simply, with deep discharges, the loss of capacity accelerates significantly compared to antimony batteries (the reasons are described at the beginning of the article).
Do you think the manufacturers don't know about this? They know very well, but they will never tell you about it in detail. Their goal is completely different. Their marketing strategies are based on one single advantage of the calcium battery - it is “maintenance-free” or the absence of the need to pay attention to it, in the word “set it and forget it.” But they left the showdown about the “sores” of their “brainchild” to the discretion of their consumers, since they have nothing to say. Hence the vagueness in their service recommendations. Is it any wonder now that there is an ongoing debate about the “correct” charging of a calcium battery? The answer is obvious.
About “special” chargers for Ca/Ca
In an article about charging calcium batteries, it is simply impossible not to mention these devices. They are bought in packs by gullible car enthusiasts. After all, if they are made, then there really is a benefit from charging a Ca/Ca battery with a voltage of 16.1 V. It is quite logical that the manufacturer, knowing about the availability of such batteries, decides to produce special chargers for them. And since fools and ignoramuses simply cannot work in production, there cannot be distrust in such devices by default.
In fact, the picture is far from so rosy and unambiguous. “Special” chargers for calcium batteries have appeared on the market not because they are vital for these batteries. They were invented because there was a demand for them in the market. But demand and necessity are two different things.
And the demand for them appeared after the problem with charging Ca/Ca batteries began to be discussed on the Internet. Well, there was no way for those who bought this miracle to achieve 100% charge. The batteries stubbornly refused to boil, and the density did not reach 1.27. Smart and enterprising people saw these problems and figured out how to make money from it.
Battery not boiling? Doesn't the density increase? Here is a special charger that will boil it. And the density will increase. And if you add a couple of “smart” functions to such a device, such as desulfation, training and charging based on the swing principle, sales will go up. And convincing the user that without this charger a calcium battery will not last long is like two fingers...
Another circumstance was a great success for these same entrepreneurs. The fact is that several years ago, information appeared on the official website of one calcium battery manufacturer that their products must be charged with a voltage of 16 V. And away we go. Referring to this official information, sellers of special chargers increased their sales even faster.
And it doesn’t matter that that same information concerned only one single line of batteries, which were discontinued a couple of years later. I don’t care about the same official information provided by the same manufacturer - he explained that 16 V was needed for that batch of batteries, and today this is irrelevant.
What should we, the victims of marketing, do?
But what about those who do not want to “forget” about their battery and who do not want to put up with the fact that 2 years of operation (for the northern regions of the Russian Federation) for a calcium battery is the limit? The answer is simple - use common sense. The author of the article does not advocate one or another charging method discussed on the Internet, but proposes some kind of compromise that helps extend the “lifetime” of a calcium battery.
A compromise method for charging a calcium battery
So we come to the most important question: how to charge a calcium battery? In the source “Lead Batteries”, authors: A.I. Rusin, L.D. Khegay, 2009, pages 160–166 describe 7 ways to charge modern batteries:
Of all the above methods, the authors of this scientific work agree that from the point of view of charge completeness, the most optimal is a two-stage combined charge
. We will take this as a basis.
In the first diagram, the authors depicted the change in current and voltage during a combined charge.
The Orion Vympel-27 charger has exactly the same charging circuit. Here's the diagram:
Although the circuits are visually depicted differently, the principle underlying them is the same: as soon as the voltage reaches its maximum value, the current begins to decrease until it reaches a minimum. It turns out that at the first stage of the charge, the current strength stabilizes, and as soon as the voltage reaches its upper permissible value, the second stage is turned on - the voltage stabilizes, and the current begins to decrease. A sign of the end of the charge is considered to be the achievement of constant electrolyte density and current strength within 2 hours.
Do not take this as an advertisement for a charger, but Vympel-27 has all the minimum necessary parameters for charging 99% of calcium batteries available to car owners. For those who do not repair batteries and do not own trucks, this charger is quite enough. The purpose of this article is not to review all kinds of chargers, so Vympel-27 will be used solely as a visual aid for charging a calcium battery. Every car enthusiast has the right to use any other chargers.
What's the compromise?
What is the compromise of the proposed method of charging a calcium battery? The compromise is as follows: we will not charge the battery at any one voltage (14.8 or 16.0 volts), but will divide the charge into 2 stages. Before charging, the battery must be kept for at least 8 hours at room temperature (20-250C). Also be sure to check the electrolyte level. It should cover the upper edge of the plates by 25-35 mm (for Akom batteries). If it is lower, bring it up to normal by adding distilled water.
1st stage of charging
At the first stage, we will use an automatic charging algorithm, in which the Vympel-27 charger (hereinafter referred to as charger) limits the maximum voltage to 14.8 volts. This will be the main stage of charging the battery, which will charge the battery to approximately 90% of its actual capacity.
So, on a charger DISCONNECTED from the network (220V), we connect the positive terminal to the positive terminal of the battery, and the negative terminal to the negative terminal. Then we set the switch to position 14.8 V and a current equal to 0.1 of the rated capacity. For example, if the battery is 60 Ah, then the current strength is set to 6A. Next, we connect the charger to a 220 V network. After connection, charging will begin automatically.
When the current drops to approximately 0.6A and does not decrease further, the first stage of charging can be considered complete. Next, disconnect the charger from the 220 V network, leaving the device connected to the battery. For what? The fact is that during charging, when the charger is connected to a 220 V network, the automation does not allow changing the current value.
2nd charging stage
What is the 2nd stage of charging needed for? In our case, we will charge a calcium battery manufactured by JSC Akom, and the instructions for charging batteries from this manufacturer directly state that in order to effectively and fully charge a battery manufactured using Ca/Ca technology, the charger must provide a charging voltage of 16.0 B. Below is a screenshot from the instructions.
Charger Orion Vympel-27
On the Vympel-27 charger, just for these cases, there is a manual “recharge” mode at 16.0 V. Therefore, we set the switch to the 16.0 V position (the charger remains disconnected from the 220 V network), and limit the current to 2A (1/30 of the nominal capacity). After setting the necessary parameters, we connect the charger to a 220 V network and continue to monitor the charge.
At a voltage of 16.0 volts on the electrodes of a calcium battery, electrolysis of water begins (decomposition into hydrogen and oxygen). Simply put, the battery begins to “boil”. And the closer the end of the charge, the stronger the “boiling” will be. This is a normal process that promotes mixing of the electrolyte and ensures a more complete “dissolution” of the lead sulfate deposited on the plates as a result of the previous discharge.
Next, when the current stops decreasing, we keep the battery in recharging mode for another 2 hours and disconnect the charger from the 220 V network. That’s it, charging the calcium battery is complete. This charging method definitely causes some harm to the battery, but it is the most gentle and represents a kind of compromise, implying the choice of the lesser of “two evils”.
Control measurements
After completing the charge, use a hydrometer to measure the density of the electrolyte. If the density is 1.27-1.28 g/cm3, then this corresponds to a 100% charge. If the electrolyte density remains low (1.22-1.23 g/cm3), then this indicates deep sulfation of the electrodes, resulting from chronic undercharging of the battery or due to deep discharges. There are definitely chances to restore the capacity of such a battery, but such measures, for example, restoring the capacity of a calcium battery by conducting control training cycles (CTC), will be discussed in a separate article. And now …
Briefly about voltage and density
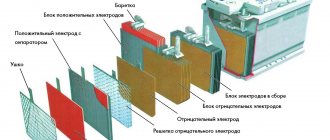
A typical representative of a maintenance-free calcium battery
These two parameters allow you to assess the degree of charge of a battery with liquid electrolyte. And in this particular case, we need them in order to understand whether we have charged the calcium battery correctly. Therefore, let's start with how to navigate these parameters.
Voltage
This refers to resting voltage . It can be measured correctly only after the battery has stood without any work for at least 8-10 hours (without charging or discharging). During this time, all electrochemical processes in it will gradually die out. And the tension stabilizes. Very often they forget about this nuance and measure it at random. As a result, the voltmeter shows inflated data, misleading the car enthusiast.
If you measure the rest voltage correctly, then based on the obtained indicators you can draw conclusions about the state of charge of the battery. The same applies to a dead battery. Whatever state it is in, the resting voltage will “tell” how many percent it is charged. This can be determined using a simple sign.
| Quiescent voltage (V) | Charge(%) |
| <11,90 | 0 |
| 11,95 | 10 |
| 12,00 | 20 |
| 12,05 | 30 |
| 12,15 | 40 |
| 12,20 | 50 |
| 12,30 | 60 |
| 12,40 | 70 |
| 12,50 | 80 |
| 12,60 | 90 |
| 12,70 | 100 |
| >12,71 | Incorrect measurement |
As real experience in using calcium batteries shows, the transition to a resting state often does not occur after 8-10 hours of inactivity. Sometimes you need to wait a little longer. For example, the author of this material regularly charges the calcium battery of his car with small currents for two or three days in a row. When the charge stops in the evening, in the morning the voltage at the terminals is usually more than 13 volts. This means that the state of rest has not yet arrived.
Most likely, it does not occur for so long due to the fact that the day before there was a long charge with a low current. After all, when the battery is charged regularly from the generator, the voltage is always adequate in the morning. That is, within the framework of what is listed in the plate above. From this we can conclude: the longer the calcium battery was charged, and the lower the currents, the longer it is necessary to wait for the onset of a state of rest.
Density
This is a hydrometer - a device for measuring the density of electrolyte in a battery.
Let us immediately note that for owners of calcium batteries without plugs, this information is useless. If you have a serviceable type battery, then you definitely need to know what the density of the electrolyte is and how to use it to determine the degree of charge of the battery. It is measured with a hydrometer. You can also determine the percentage of charge using the table. Although with some reservations related to the main feature of calcium batteries. But more on that a little later. In the meantime, here's the sign.
| Density (g/cm3) | Charge(%) |
| <1,12 | 0 |
| 1,14 | 10 |
| 1,15 | 20 |
| 1,16 | 30 |
| 1,18 | 40 |
| 1,19 | 50 |
| 1,20 | 60 |
| 1,22 | 70 |
| 1,23 | 80 |
| 1,25 | 90 |
| 1,26 | 100 |
| >1,26 | Density is higher than normal |
The advantage of density compared to resting voltage is that you don’t have to wait 8-10 hours to measure it. Correct readings can be taken at any time. Even while charging the calcium battery. But let us repeat that with some reservations, which we will now begin to analyze.
Dedicated to opponents of “boiling” calcium batteries
In anticipation of a barrage of criticism from “supporters of 14 volts,” I would like to draw attention to some scientifically proven facts. First, let's look at what underlies the arguments that 14-volt proponents make.
MYTH: “Boiling” is the greatest evil for the battery
Often in favor of this theory you can hear statements like this: “Why do something that the manufacturer so diligently wants to avoid?” Some supporters of 14 volts naively believe that the manufacturer, through incredible efforts, managed to get rid of the most important enemy of the battery - “boiling”, and that it was “boiling” that led to the destruction of the electrodes in lead-acid batteries throughout the 160 years of their existence. Finally, we have done what car owners have been waiting for! Finally, the battery has become “perfect”! As sad as it may be, the situation is the opposite...
FACT: The use of “calcium” technology in production, which allows you to avoid “boiling” the battery, is nothing more than a marketing ploy. Yes, yes, nothing more! This is just a market reaction to the emerging trends of modern automotive society. Car owners WANT “set it and forget it” batteries. Don't want to mess with the battery? No problem - get a “calcium miracle”!
For more than 70 years, no one needed “calcium” technology, because it did not “fit” into the market economy, but today its “finest hour” has come. The only advantage of a calcium battery over an antimony battery is that there is no need to closely monitor the electrolyte level. It is this feature of “calcium” technology that forms the basis of the marketing campaigns of all manufacturers. And this is done not because “boiling” is evil, but because people simply WANT it. The second most important advantage of “calcium” is its low self-discharge, and this is probably where all its advantages end.
EVIDENCE: Electrolysis of water or so-called “boiling” is a common operating process in the operation of a lead-acid battery - this is stated in all textbooks and scientific works published over the past decades. Yes, during the process of electrolysis or “boiling” there is a slight shedding of the active mass, but without “boiling” all the lead sulfate on the plates will not dissolve and the battery charge will be incomplete. Each battery is designed for a certain number of cycles (discharge/charge) and with each cycle there is a kind of “wear” of the battery plates, characterized by shedding of the active mass and irreversible loss of capacity. So what now? Do not use the battery? Let's draw an analogy with the braking system of a car. Imagine being told: “When you are driving a car, do not press the brakes, otherwise the brake pads and brake discs will wear out at this moment.” What do you think of this statement?
OBJECTION-1: Some supporters of 14 volts will exclaim: “Don’t you understand that boiling at 14.4 V (for antimony batteries) is not the same as boiling at 16.0 V (for calcium batteries). Boiling at 14.4 V is good, but boiling at 16.0 V is already evil! It is at 16.0 V that intense melting of the active mass occurs!!!”
ANSWER: Let's find out the opinions of experts in this field. First source: “Operation, maintenance and repair of lead-acid batteries”, authors: V.I. Bolotovsky, Z.I. Weisgant, 1988, p. 96. Below is a screenshot.
Have you noticed what voltage needs to be applied to one battery cell? One can needs 2.7 volts, and six cans (2.7 x 6 = 16.2 volts).
If you think that this book is very “shaggy” and the data does not correspond to modern realities, then let’s turn to a more recent source: “Lead Batteries”, authors: A.I. Rusin, L.D. Khegay, 2009, pp. 162,163. Screenshot below.
As you can see, first there is a comment that when one battery cell reaches 2.4 volts, electrolysis begins. If we multiply 2.4 x 6 = 14.4 volts, we begin to understand that we are talking about antimony batteries, because it is in them that electrolysis begins at a voltage of 14.4 volts. But the most interesting thing is below. At the end of the charge, the voltage increases to 16.2 volts (2.7 x 6 = 16.2 V).
What do we get from this? And the fact is that both at 14.4 V and at 16.2 V, lead batteries feel great.
OBJECTION-2: A calcium battery can be easily charged at 14.8 V.
ANSWER: Absolutely right, although it’s not calm, it’s charging. You will only “drive” it up to 100% charge for a week, and then only on condition that the battery does not have deep sulfation. In reality, no sane driver will charge his battery for 7 days. It’s another matter when there is no choice, the battery is dead, for example. Only in this case will the driver have to allocate enough time to try to “revive” his battery.
OBJECTION-3: To bring the density of a calcium battery to the “norm” (1.27 g/cm3), after charging at 14.8 V, it is enough to shake the electrolyte. Or, immediately after charging, put the battery in the car and drive it over bumps. Then the more concentrated electrolyte, which is in the lower part of the cans, will mix with the less dense upper layers and everything will be OK.
ANSWER: The very purpose of the CHARGING process is to "convert" ALL lead sulphate (PbSO4) produced during DISCHARGE back into lead dioxide (PbO2) on the positive plate and into lead metal sponge (Pb) on the negative plate. As a result of these chemical reactions, in addition to the “transformations” described above, water is consumed and sulfuric acid is formed. The following illustration schematically shows what happens to the electrolyte during a charge limited to 14.8 V.
What do we see here? During charging, the sulfuric acid formed on the positive electrode flows to the bottom of the battery under the influence of gravity, displacing water to the surface. This process is called stratification or stratification of the electrolyte. As a result of electrolyte separation, lead sulfate remains “undissolved” on the bottom of the battery plates because there is not enough water for the chemical reaction, since almost all the water is in the upper layers of the electrolyte. So what, you ask?
And the fact is that the battery will remain 100% uncharged, because part of the sulfuric acid remains “packed” in lead sulfate on the bottom of the plates.
The only solution to this problem is to constantly stir the electrolyte during the last stage of charging so that the water entering the lower part reacts and “dissolves” the lead sulfate until it is all converted to sulfuric acid. Only then can the battery be considered 100% charged. It is precisely the “boiling” while charging the battery that performs this stirring role, but... at 14.8 volts, the calcium battery practically does not boil, only at 16.0 V.
From here we can draw a logical conclusion: shaking the electrolyte at the end of the charge is not enough. You simply mix the electrolyte, equalize the density, but the lead sulfate will remain on the bottom of the plates! The battery charge will not reach 100%.
OBJECTION-4: But the density of the electrolyte shows 1.28 g/cm3 - which means all the sulfate has dissolved!
ANSWER: There are no miracles in our time. Check the electrolyte level. Most likely it is below normal. Hence the high density. Let me give you a real example: a calcium battery has never been serviced for a year and a half since purchasing the car, the mileage of the car at the time of the battery inspection was 30,000 km, the operation was daily. The condition of the battery at an electrolyte temperature of 210C was as follows:
- Open circuit voltage (OCV) at terminals: 12.84 V;
- Electrolyte density in all banks: 1.28 ±0.01 g/cm3;
It would seem that everything was fine, but when we looked into the jars, it turned out that sulfation of the plates was thriving in all the jars. The electrolyte level was 10-15 mm above the plates, instead of 25-35 mm recommended by the manufacturer (AKOM JSC), which means that more than 400 ml of distilled water was missing. So much for excellent density! Bring the electrolyte to the required level and the picture will no longer be so rosy.
How to properly charge a Sa/Ca battery
- If the battery in your car is not fully charged (the reasons may vary: low outside temperatures, short and infrequent trips, problematic alternator, etc.), you need to charge it using a regular charger
- The charge voltage should be in the range of 14.4-15V
- The charge current should be no more than 10% of your battery capacity
- Algori standard for lead-acid batteries; charge with constant current to a threshold voltage, then charge with constant voltage with a decrease in the charge current.
- “Boiling” calcium batteries is strictly contraindicated. Since, at best, it leads to a decrease in the technical characteristics of the device, and at worst, to failure of the device.
- To achieve a more “dense” charge, better dissolution of sulfates and increased service life, it is necessary to charge the battery with the lowest current value.
Nowadays there are many fakes on the market. To distinguish a high-quality battery from a fake, and also to understand whether the device in front of us is original or not, you need to pay attention to the markings. The following characteristics must be indicated on the battery case:
- starting current
- voltage value
- rated capacity value
- release date of this device
- detailed information about the manufacturer
Everyone has the right to choose their own charge voltage and current. But have you noticed that we are not talking about a charge of 16 volts or more? These batteries are charged in the same way as lead-acid ones.
Summarizing
What conclusions can be drawn regarding the proper use of calcium batteries? Let's summarize our reasoning.
- Avoid deep discharges, because restoring the capacity of a calcium battery after such discharges is extremely problematic. Therefore, do not turn the starter until it stops cranking the engine (especially in winter) and do not forget to turn off the lights, which can easily discharge your battery in a day;
- Maintain the battery at least once every six months or at every maintenance (adjusting the electrolyte level and charging with a stationary charger).
- Be sure to recharge the calcium battery at 16.0 V to convert any remaining lead sulfate to sulfuric acid.
On this, perhaps, it’s time to end the article. Thank you for taking the time to read such a lengthy material.
If you have any questions, ask in the comments, I will help as best I can. If you think that I am wrong about something, please support your arguments with scientifically proven facts, and not with unfounded statements and “rich personal experience.”
Features of operation
The characteristics of calcium batteries, on the one hand, are their competitive advantage, and on the other, they require special operation of the device. Below are the basic recommendations for the use and maintenance of batteries of this type (some of them have already been mentioned, but require clarification, while others may be new to some):
- If a car with a new calcium battery is used to cover short distances, then it must be charged at least once a month. In other cases, recharging is needed half as often. The automotive spare parts market offers the widest selection of chargers for any budget. Some models are simple to implement and require certain knowledge, but there are also those that a completely ignorant driver can handle.
- Most manufacturers recommend charging a Ca/Ca battery until its voltage reaches 14.4 V. It is desirable that the current be no higher than 10% of the declared capacity.
- If the voltmeter shows 12 V, the device needs to be charged immediately. Otherwise, the car may simply not start next time.
- Many motorists who have not learned how to charge a lead-calcium alloy battery, out of habit, carry out a control and training cycle with it, that is, they completely discharge and charge the battery. This procedure has a detrimental effect on batteries of this type, since when the charge is weak, the plates become sulfated.
- Once again, it is worth recalling that “boiling” is strictly contraindicated for calcium batteries.
- Even if you purchased a serviceable calcium battery, you cannot accurately measure the density of its electrolyte using a hydrometer. Firstly, as already mentioned, the density may be different in different “banks”. Secondly, modern batteries are designed in such a way that their electrolyte can separate into denser and more watery phases. Consequently, in the zone acceptable for measurement, the liquid will have a low density.
- Today, you can easily encounter counterfeits on the automotive spare parts market. How to check the battery? Quite simple: a good battery must be fully labeled with data such as type, voltage and rated capacity, starting current, production date, detailed information about the manufacturer (preferably with an address). As for the instructions, its presence in the battery kit is not at all necessary. The fact is that in the West, batteries are practically not sold at retail. If necessary, people simply go to a service center, where they receive qualified service or battery replacement.
Since the battery is one of the most important parts in the design of a car, its choice should be taken seriously. There is no point in saving too much, especially when it comes to calcium batteries. Varta, Bosch, Delkor, Topla are companies that have proven themselves well in the market and whose products it is recommended to pay attention to first.
Hybrid Battery Maintenance
Hybrid devices are low-maintenance, but once every 3 months you will have to remove the cover and check the electrolyte level. On average, you will need to add 0.5 liters of water at a time.
If you regularly check the volume of electrolyte and its density, then the battery will not have a problem. Most models have marks indicating what the electrolyte level should be. Some manufacturers equip their products with an indicator.
Maintenance includes cleaning white deposits - lead sulfate. This is normal and is a result of a chemical reaction associated with lead.
If the acidity of the composition is incorrect, the destruction of the plates can be accelerated, and no repair will help. If desulfation is carried out periodically, this process can be stopped.
Technologies involved
The technology for creating batteries with the addition of calcium is completely different from the stages of developing batteries with the addition of antimony.
When making plates from lead, casting is not used, since when the metal melts, calcium begins to burn out.
Plates with the addition of Ca are carefully stamped, so there is no fear that the metal will begin to evaporate.
The performance of such a battery is no lower than that of an antimony-doped battery, however, to charge the battery, a charger with a high pressure rating should be used.
What is a hybrid battery
The semantics of the word “hybrid” already suggests that we are talking about a combination of different directions. In this case, this is a crossing of technological processes for the production of batteries, based on the use of the electrolyte properties of two chemical elements - calcium and antimony.
About half of the plates in a hybrid battery are lead-antimony, the other half are lead-calcium. The marking on car batteries Calcium Plus or Ca+ means that the product is produced using hybrid technology. Sometimes it’s written on the case: hybrid Calcium +.
Charging algorithm for a battery device C/AC/A
If a device with regulation is used for charging, then the voltage level should be 16 Volts, and the current indicator should be equal to one tenth of the battery volume.
In such conditions, charging will last about 10 hours. When connecting a battery to an automatic charger, polarity is important.
If, during discharge, processes are started that lead to the coating of battery plates with lead sulfate, and the charger is equipped with a training mode, then this function should be activated.
How to choose a memory
In order to avoid problems with recharging the power source, you should be responsible when selecting a charger:
- The charger should produce the optimal voltage for recharging - 13.8-14.2 V.
- The requirements for the maximum current output, which determines the charging time, are determined by the battery capacity specified by the manufacturer. Powerful charger will charge a more powerful battery.
- The charger must provide for step-by-step adjustment of the output voltage.
- The transformer device must be equipped with a maximum current limiter.
- It is best to purchase a model with an automatic control unit to avoid setup errors.
- Ideally, the charger can block the charging process if the terminals are connected incorrectly and have protection against voltage surges in the network.
Rechargeable charger options charge the battery for a long time, but sparingly. Start-charging models operate in accelerated mode and have a quick recovery function. They carry out desulfation and restore the factory capacity of the battery.
Best before date
According to the warranty, C/AC/A batteries will serve properly for five years, but if deep discharges are avoided, such batteries can operate and maintain the required level of starting pressure even after 8-10 years.
Violation of the rules of use may result in the product not working for even a year. If it turns out that the cause of the breakdown was the buyer's negligence, no compensation will be paid.