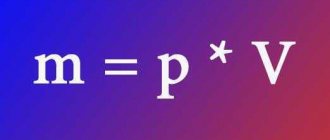Today, the operation of almost all vehicles - cars, airplanes, helicopters, combines and various mechanical devices - depends on fuel. In order to correctly keep records of the issuance of petroleum products, you will need to know how to convert liters of gasoline into kilograms, as well as tons. Do not forget that this is a measure, a definition of volume, and kilograms or tons mean the weight of the substance. Calculations are an important component in the work of accountants and storekeepers when accounting for the fuel mixture. Conversion of units of measurement, arithmetic, which implies the need for certain knowledge, formulas and numbers.
How many grams are in 1 liter of milk?
The density of milk can change depending on temperature and pressure. Question: How many grams are in 1 liter of milk? Answer: 1 gram of milk = 0.000971 liters, 1 liter of milk = 1030 grams.
Interesting materials:
How to distribute the Internet over a local network via Wi Fi? How to distribute Internet from iPad to iPhone? How to distribute Wi-Fi from a tablet? How to divide a number by 10? How to separate a mixture of alcohol and water? How to split a page into two PDF parts? How to smooth out Eco-leather on a stroller? How to smooth a genuine leather skirt? How to smooth a leather sheepskin coat? How to iron a leather jacket?
How does the density of a substance affect the calculation of its mass in tons?
The number of cubic meters of petroleum product depends on the ratio of mass to volume. The value, in turn, depends on the quality and manufacturer. A high value of the indicator indicates high-quality raw materials. We have already figured out above that the volume of fuel in liters increases during heating; at sub-zero temperatures, the fuel turns into a jelly-like substance and becomes thick. When recalculating, it will be necessary to take into account the influence of temperature, as well as other influencing factors. To perform specific calculations, you will need to use professional tables containing the required indicators, taking into account the effect of temperature on the fuel.
This volume is in spoons, glasses, jars, etc.
Please note that the weight is calculated based on the volume of the containers and we do not in any way guarantee that they contain the specified volume, however, for approximate measurements in everyday life, the calculated weight is quite applicable.
To determine a more accurate weight, you should use a scale!
Also, keep in mind that some substances can have a destructive effect on the indicated containers and cannot be contained in them in real life.
Tea spoon
18400 pcs.
Tablespoon
5111.1 pcs.
Cup
368 pcs.
Jar 1 l
92 pcs.
Bottle 1.5 l
61.3 pcs.
Jar 3 l
30.7 pcs.
Canister 20 l
4.6 pcs.
This is interesting: How to replace the boot on a VAZ 2109 grenade
How to calculate the density of petroleum products
When there is no special device, then calculations should be made based on the information in the product passport and the indicators in the table of average temperature corrections. To obtain the necessary data, it is enough to perform the following basic calculations:
- In the documentation we find its density indicator at +20 Celsius.
- We measure the temperature of the product.
- We calculate the difference between the obtained result and +20 Celsius.
- Round the resulting value to a whole number.
- In the table we find the correction for deviations in accordance with the passport value at +20 Celsius.
- We calculate the product of the correction for the temperature difference.
- We combine the result with the passport value of density at +20 or subtract it if the temperature is higher.
There is nothing complicated. All mathematical operations are a school curriculum; there is no need to resort to laboratory research.
Table of the influence of temperature on the density of kerosene, kg/m3
Temperature corrections were used to calculate volume changes for different grades of kerosene at different temperatures.
| -20°C | -10°C | 0°C | 10°C | 30°C | 40°C | 50°C | |
| TS1 | 811 | 803,76 | 795,84 | 787,92 | 772,08 | 764,16 | 756,24 |
| RT | 810,2 | 802,15 | 794,1 | 786,05 | 769,95 | 761,9 | 753,85 |
| T1 | 849,08 | 841,56 | 834,04 | 826,52 | 811,48 | 803,96 | 796,44 |
| T2 | 788,24 | 779,93 | 771,62 | 763,31 | 746,69 | 738,38 | 730,07 |
| T6 | 869,48 | 862,36 | 855,24 | 848,12 | 833,88 | 826,76 | 819,64 |
| Lighting | 868,48 | 861,36 | 854,24 | 847,12 | 832,88 | 825,76 | 818,64 |
| KO30 | 821,12 | 813,34 | 805,56 | 797,78 | 782,22 | 774,44 | 766,66 |
| KO22 and 25 | 835,6 | 827,95 | 820,3 | 812,65 | 797,35 | 789,7 | 782,05 |
| KO20 | 859 | 851,75 | 844,5 | 837,25 | 822,75 | 815,5 | 808,25 |
Heat always causes expansion. This means that the same substance will take up more space. Reference values are given based on a correction factor.
If you need to calculate more accurately, you can use the correction factors below. They show how much the volume of 1 cube changes when the temperature changes by 1 degree Celsius.
- TC1 – 0.792
- RT – 0.805
- T1 – 0.752
- T2 – 0.831
- T6 – 0.712
- Lighting – 0.712
- KO30 – 0.778
- KO22 and 25 – 0.765
- KO20 – 0.725
It is important to remember that if the temperature drops, then we add the value multiplied by the number of temperature drops; if it rises, then we subtract the multiplied value. Why is that? When a substance cools, it contracts; when it expands, it increases in volume.
Suppose a cube of T6 kerosene at +30 degrees weighs 833 kg. How much will the cube weigh at 0°C?
Falling to 0°C it will lose 30°C, which means you need to multiply 30 by a coefficient and add it to 833. Remember, a coefficient is a number that changes the volume of a cube when its temperature changes by 1 degree.
How exactly?
The temperature drops: Weight of the cube + (Temperature * coefficient) = 833 + (30 * 0.712) = 854 kg of T6 kerosene in the cube, at a temperature of 0 degrees.
If the temperature rises from +30 to another 30, then you will need to subtract. Because the substance will expand and take up more space, which means less kerosene will fit into the cube.
There may be confusion due to unfamiliarity with calculating these values, but after reviewing the calculations several times, there will be more clarity.
Barrel of kerosene
- A barrel is 158 liters. It is known that the average weight of kerosene is 0.8 kg per liter.
- This means: 158 liters of kerosene weigh on average 126 kg (158 * 0.8).
- Which is 0.126 of 1 ton.
- There are 7.9 barrels of kerosene in a ton.
Following the example of these calculations, you can calculate for a specific brand of kerosene, based on the data in the tables above.
What does the current fuel density depend on?
The current density of diesel fuel depends on its brand and temperature. You can find out its density at a temperature of 15 or 20 degrees Celsius in the quality certificate.
The higher the temperature of the oil product, the less 1 liter will weigh, and the volume of a kilogram of fuel will increase.
When converting units of measurement, the formulas are used: m = p * V and V = m / p.
To check whether the density of diesel fuel corresponds to the data specified in the quality passport, you need to take a liter of diesel fuel, weigh it and place it in the temperature environment in which the manufacturer conducted density tests: +150C or +200C. The exact weight of the fuel must be divided by its volume. We get the current density and compare it with the passport data.
Features of converting gasoline of different brands from liters to tons
To carry out the arithmetic calculation correctly, you will need to know the table value of the density of the fuel grade. The average density of fuel at room temperature is 0.75 g/l. For detailed measurements, it is necessary to use a correction for ambient temperature in the calculation. This will come in handy when the temperature limits go beyond the accepted limits of 15-20 C. To make the task easier and not waste time on formulas on the Internet, an online calculator program has been developed that performs complex calculations automatically. In the form you need to indicate:
- type of petroleum products;
- volume, mass;
- cost per liter;
Densities of different brands of fuel:
- AI-76 - 1398.6 g/cm3
- AI-80 - 1398.6 g/cm3
- AI-92 - 1360.5 g/cm3
- AI-93 - 1360.5 g/cm3
- AI-95 - 1307.2 g/cm3
- AI-98 - 0.765 g/cm3
Converting any type of fuel from liters to tons is not such a difficult job. It is important to take into account all parameters, external factors and amendments when calculating.
Compression ratio and risk of detonation
When considering an internal combustion engine (ICE), it is represented by the ratio of the total volume of the cylinder to the size of the combustion chamber. Modern engines are characterized by a coolant ratio in the range from 8 to 14. Increased compression rates force the use of high-purity fuel, otherwise detonation is possible.
An increase in coolant is provided to increase the internal power of the car, the efficiency of the heat engine increases and due to this the overall fuel consumption is reduced. Fuel must be refueled in accordance with these parameters, so the debate about whether AI-92 or AI-95 is better is not appropriate. You need to look at the ratio of coolant to fuel grade (presented in the table).
| Compression ratio | Recommended fuel brand |
| 8-10 | AI-92 |
| 10-12 | AI-95 |
| 12-14 | AI-98 |
| 14-16 | AI-100 |
| 16-18 | AI-103 |
| Over 18 | AI-106-109 |
It is especially important to follow similar recommendations for owners of turbocharged cars and use fuel no lower than AI-95. If this rule is not followed, the fuel may spontaneously ignite, causing small explosions. Detonation itself is dangerous for the car.
The rings may collapse, and the piston and gasket between the block and its head may burn.
Detonation itself is dangerous for the car. The rings may collapse, and the piston and gasket between the block and its head may burn.
I would like to note the fact that in the USA they use a special method for identifying the anti-knock resistance of AKI. Therefore, when operating, have the following compliance indicators:
- AKI 87 = AI-92;
- AKI 91 = AI-95;
- AKI 93 = AI-98.
Density Tables
The density of a substance is one of the most important physical indicators of an object. It is equal to the ratio of volume to its mass, expressed in kilograms. This characteristic depends on temperature. The higher the temperature, the lower the indicator, and vice versa.
This process is directly related to the phenomenon of thermal expansion. That is, with stable body weight, the volume of the substance increases. There is no need to determine this physical criterion yourself. To do this, just use the density tables.
For example, following them, you can determine that the fuel has the following indicators:
AI-76 - 715 kg/cu.m. meter;
| № | Helpful information |
| 1 | AI-92 - 735 |
| 2 | AI-95 - 750 |
| 3 | AI-98 - 776.5 |
| 4 | summer diesel - 860 |
| 5 | winter diesel - 840 |
| 6 | Arctic diesel oil product - 830 |
Fractional composition of gasoline
All these indicators can only be determined in a laboratory setting. Therefore, it is not possible for consumers to determine the quality of fuel by analyzing the chemical composition.
However, the fluctuations are insignificant. Therefore, standard tables are quite suitable. It is better if the density tables at the enterprise are approved by internal order, and they are the ones that will be used for permanent work. The fact is that in different sources on the Internet, the indicators vary insignificantly.
Mass of a liter of gasoline?
It is reliably known that gasoline is conventionally divided into several categories. So this substance exists under the markings A-76, A92, A-98, A95. They may differ in some properties from each other.
Accordingly, the weight of 1 liter of gasoline is different for each of these categories, even despite the identical displacement. By simple mathematical calculations it has long been found out how much weight 1 liter of each brand of gasoline weighs. So, if we talk about the A-95, then it weighs 750 grams.
Accordingly, 2 liters of A-95 will weigh one and a half kilograms, and so on. At the same time, the A-80 weighs 30 grams less with the same volume of 1 liter. It is noteworthy that the mass of the A-76 is exactly the same as that of the A-80, but only if the temperature of this brand of gasoline does not exceed 20 degrees Celsius.
The A-98 is considered the heaviest. Its mass is as much as 780 grams per volume of 1 liter. The second heaviest aircraft is the A-92. There are 760 grams per liter.
So we found out how many grams in 1 liter of gasoline are available in each of its most popular brands. However, we must not forget about the other characteristics of gasoline. Thus, the specific gravity of gasoline can change on its own, regardless of the displacement, and all due to the influence of ambient temperature. So, the higher the value the thermometer shows, the lighter the mass of gasoline will become.
There are even special laboratories where the same amount of a substance is placed in different environments, and then they compare how its mass has changed. So, if the ambient temperature is +16, then 1 liter of A-92 weighs 765 grams. It’s strange, because according to the measurements described above, 1 liter of A-92 is equal to 760 grams.
Why has the mass now become a little heavier? It's all about the temperature of the surrounding space. Therefore, no matter how many times the measurement is carried out, the new result may always be slightly different from the previous one. A more complete picture emerges if you purposefully take measurements with a consistent increase or decrease in temperature. Then you will be able to correctly determine what the density of gasoline is.
In the modern world, people buy gasoline, paying attention to the displacement last. Most of them fill the car's gas tank full. At the same time, the amount of displacement may vary depending on the type of car and its configuration.
They also often buy gasoline in cans so that they always have a reserve in case the gas tank is empty and the nearest gas station is too far away. So, if you have a 19-liter canister, then you can easily find out how much it weighs when empty and when filled with a substance.
If a 19-liter canister is filled to the top with gasoline, then its weight is 16 kilograms 325 grams. As for the empty container, its mass is 2,550 kilograms. If you consider that the weight of one liter of gasoline is 725 grams, then you can easily calculate everything. First, subtract the empty weight from the weight of the full canister. It will be 16.325 – 2.550. The result is 13.775 kilograms.
This means that a 19-liter canister holds 13.775 kilograms of gasoline. It is easy to check this value. It is enough to divide the resulting net weight of gasoline by the mass of 1 liter. That is 13.775 kg / 0.725 kg. It will turn out to be just 19.
Such simple mathematical calculations allow you to find out the mass of gasoline in any canister, regardless of its capacity.
And yet, someone will probably ask why convert one unit to another? What is the use of knowing how many liters of gasoline are needed to make 1 kg? In fact, they do all this for a reason. For those who use these flammable substances, this translation is of particular importance.
In particular, accountants who work with gas stations, logistics companies, etc. constantly encounter it. If you take into account and calculate how much and in what quantities certain bulk and liquid substances, such as gasoline, are stored in the enterprise’s warehouses, then it is often necessary to convert the values.
Thus, converting liters into kilograms is necessary for those who fill out reports, and to make it easier for them to carry out financial calculations and make payments for the wholesale sale of a substance. First of all, this is due to the traditional method of delivery - in a tank with a certain capacity.
However, accounting is always carried out in bulk. In addition, if the product is sold in bulk, it is easier to count everything in tons. That’s why it’s worth knowing how much 1 liter of gasoline weighs, depending on its brand. Otherwise, the calculations would be much more complicated, which could lead to more frequent errors in this arithmetic process.
Those who change from a normal machine will be very disappointed. Review of Lada Vesta Cross
In traffic jams the car jerks a lot.
When you press the gas harder, it jumps forward. SW Cross 1.8 Multimedia, robot. Purchased three weeks ago. Traveled 700 km. Gasoline consumption is 9.8 l/100 km (80% of highway driving is on a cruise of 110 km/h at exactly 3000 rpm). Anyone who switches from a normal machine will be very disappointed, as happened to me.
In traffic jams the car jerks a lot. When you press the gas harder, it jumps forward. You have to keep a greater distance. I haven’t yet understood the logic behind enabling the “creeping” mode. If you use this mode while driving, and press the gas, catch up with the car in front, and brake, then when you release the brake, the “creep” does not turn on.
You have to frantically press the brake pedal several times to activate it. On the highway, during smooth acceleration, dips are felt when switching from 1 to 2 and from 2 to 3 speeds.
Suspension and handling are very good. I don’t notice any creaks or any extraneous sounds. Noise protection is good. Heated seat buttons with 3rd adjustment, a separate button for heated front glass available. There is interior lighting in the niches under the radio, in the feet and in the doors. They even put a trunk net measuring 35 x 15 cm
Climate control. At 30 degrees in a traffic jam, I had to turn off the automatic control and turn on the fan to maximum because it wouldn’t turn off and it would get hot.
Wheels and tires. The photo shows the result of hitting a 5 x 5 cm block with the rear wheel at a speed of 80 km/h.
The wheels and tires are very soft, so in my situation both components are scrapped. It is impossible to find original summer tires (Pirelli 205/50 R17 89V) at dealers and on the open market!!
PS The reverse mode started to stick. The camera turns on and when you press the gas the car stalls. The lever is in neutral, you twist the key, the rear view camera immediately turns on, the car does not start (the starter does not turn). After 20 minutes of parking without a key, the glitch was reset.
Something like this.
Read also: Surprisingly, AVTOVAZ met halfway: continuation of the story with a jammed engine 2 days after the purchase of Vesta Cross
www.lada-vesta2.ru
Transition formula
It is known that body weight is found by arithmetic multiplying the volume of a petroleum product, measured in liters, and its density. Mass is understood as a physical quantity that characterizes the performance of a body: its inert and spatial properties in space.
Oil and diesel fuel in this case are determined using special density reference tables. For the final calculation of the mass of combustible fuel in tons, you can use a formula known from a school physics course.
m = V × ρ, where:
- m is the mass of the product, measured in kilograms;
- V is the volume of gasoline. Measured in cubic meters;
- ρ is the density of the substance. We determine it using special tables.
For calculations we use the generally accepted SI system. It is a worldwide system for measuring physical quantities. Also, do not forget to make the necessary conversions into units of measurement:
- liters will need to be converted to cubic meters using the formula: 1 liter is 0.001 cubic meters. meters;
- convert tons to kilograms, use the formula: 1 ton = 1000 kg.
Read also:
How is E85 gasoline made?
View this post on Instagram
Publication from REGIONTRANS (@regiontr.ru) January 10, 2022 at 12:53 PST
Government standards applicable to gasoline
Car owners often wonder: within what limits should the density of gasoline be? As a rule, in the fuel used by the automotive industry, its value ranges from 700 to 780 kilograms per cubic meter. The characteristics and properties of the fuel depend on both the composition and density of the oil from which the product was produced. For example, aromatic compounds have a lower density than aliphatic ones. The parameter will fluctuate both up and down depending on the percentage of compounds included in the composition. Due to the fact that the density of fuel is not constant, experts have created tables from which it is easy to find out the permissible level of fuel density depending on storage conditions.
The oil industry is also regulated by state environmental standards. In Russia, since the beginning of 2022, GOST 32513-2013 has been in force, which sets the quality standard for modern gasoline with an octave number of at least 80 (AI-80). However, the issue of environmental protection and environmentally friendly transport is gaining new relevance every year. Consequently, at the state level, increasingly stringent requirements are being put forward for petroleum products. In 2022, the Russian Federation adopted EURO-5, a standard that regulates the percentage of heavy metals and benzene in gasoline.
Density of AI-92 gasoline
The octane number of AI-92 gasoline cannot be lower than 91. The fuel is used primarily in power units of passenger vehicles. It should appear transparent and clean. It is characterized by resistance to detonation, which is why car enthusiasts today purchase “92nd” gasoline for internal combustion engines of domestic and many imported car brands. The required parameter when measured at +15 degrees is equal to 725-780 km/m3.
Density of AI-95 gasoline
This gasoline is for cars imported from abroad. It is distinguished by high performance properties: during its production, manufacturers use technological components. The octane number should not be lower than 95. The value is measured at +15 Celsius. The standard density is 750+/-5 kg/m3.
Density of gasoline AI-100
One of the latest innovations is automobile fuel with an octane rating of 100. It is found at many gas stations, in particular at Lukoil gas stations. This is a product with additional Ecto additives. It is characterized by high performance properties and an equally high price. The standard density indicator is set in the range from 725 to 750 kg/m3 at standard +15 degrees.
How is the density of gasoline measured?
Any gasoline is a liquid mixture of hydrocarbons obtained as a result of fractional distillation of oil. These hydrocarbons can be classified into aromatic compounds, which have rings of carbon atoms, and aliphatic compounds, which consist only of straight carbon chains. Therefore, gasoline is a class of compounds rather than a specific mixture, so its composition can vary widely.
The easiest way to determine density at home is as follows:
- Any graduated container is selected and weighed.
- The result is recorded.
- The container is filled with 100 ml of gasoline and also weighed.
- The mass of the empty container is subtracted from the mass of the filled one.
- The result is divided by the volume of gasoline that was in the container. This will be the density of the fuel.
If you have a hydrometer, you can take the measurement in an alternative way. A hydrometer is a device that implements Archimedes' principle for measuring specific gravity. This principle states that an object floating in a liquid will displace an amount of water equal to the weight of the object. Based on the hydrometer scale readings, the required parameter is determined.
The sequence of measurements is as follows:
Fill the transparent container and carefully place the hydrometer into the gasoline. Rotate the hydrometer to displace any air bubbles and allow the instrument to stabilize on the surface of the gasoline.
It is important to remove air bubbles because they will increase the buoyancy of the hydrometer. Place the hydrometer so that the surface of the gasoline is at eye level. Write down the value of the scale corresponding to the level of the gasoline surface. At the same time, the temperature at which the measurement took place is recorded.
Typically, gasoline has a density in the range of 700... 780 kg/m3, depending on its exact composition. Aromatic compounds are less dense than aliphatic compounds, so the measured value can indicate the relative proportion of these compounds in gasoline.
Pycnometers are used much less frequently to determine the density of gasoline (see GOST 3900-85), since these devices for volatile and low-viscosity liquids are not stable in their readings.
Density of AI-92 gasoline
The standard establishes that the density of AI-92 unleaded gasoline should be within 760±10 kg/m3. Measurements must be taken at a temperature of 15ºC.
Density of AI-95 gasoline
The standard density value of AI-95 gasoline, which was measured at a temperature of 15ºC, is 750±5 kg/m3.
Density of gasoline AI-100
The trademark of this gasoline - Lukoil Ecto 100 - sets the standard density indicator, kg/m3, within 725...750 (also at 15ºС).
What else are they looking for?
- How much does 155 liters of liquefied oxygen weigh? 11 seconds ago
- How much does 190 liters of finely ground table salt weigh? 15 seconds ago
- How much does 115 liters of sulfur pyrite weigh? 16 seconds ago
- How much does 174 liters of crushed sodium nitrate weigh? 21 seconds ago
- How many calories are in 53 kg 248 g of 9% fat cottage cheese? 51 seconds ago
- How much does 26 liters of raw cane sugar weigh? 52 seconds ago
- How many calories are in 2 kg 16 g of morels? 1 minutes ago
- How many calories are in 3 kg 328 g of dried apples? 1 minutes ago
- How much does 5 liters of gluten flour weigh? 1 minutes ago
- How many calories are in 6 g of black currant? 2 minutes ago
This is interesting: When to turn on the headlights in the city
Other meanings
- How much does 15 liters of motor (machine) oil weigh?
- How much does 26 liters of motor (machine) oil weigh?
- How much does 66 liters of motor (machine) oil weigh?
- How much does 133 liters of motor (machine) oil weigh?
| Specific volume is the volume per unit weight of a given substance. Designated - m3/kg. Specific gravity, the reciprocal of specific volume. Its dimension: kg/m3. Molar volume is the specific volume multiplied by the molecular weight of the substance. |
| There are several methods for determining the specific gravity of substances: weighing, pycnometer, hydrometer and others, mainly the method is determined by the state of aggregation of the substance under study, its pressure, temperature and other experimental conditions. |
Density of diesel fuel in summer and winter
Specific gravity of diesel fuel (diesel fuel)
The fuel used in summer and winter differs in its main characteristics.
For winter, fuel that has the following indicators is suitable:
- low density;
- low viscosity;
- does not freeze at a temperature of -45O.
Low viscosity density allows the fuel not to turn into a gel and circulate freely inside the internal combustion engine. In cold weather, the use of high-density fuel is strictly prohibited in order to avoid problems with the filter system.
Summer fuel is characterized by:
- high density (not less than 0.86 kg/l);
- difficult to ignite (withstands temperature increases up to +45°C).
When winter fuel is used in the summer, engine power decreases and smoke levels increase.
The best time for summer fuel is April - September, if the temperature does not drop below -4°C. Otherwise, the diesel fuel will become cloudy, and when the temperature drops another few degrees, it can lead to problems with engine operation.
Geographical position
Bashkortostan is located on the border of Europe and Asia. The territory of the republic is just over 143 thousand square meters. km and covers part of the East European Plain, the mountain system of the Southern Urals and the Trans-Ural uplands. The capital of the region, Ufa, is the largest populated area of the republic; the other cities of Bashkiria are much inferior to it in terms of population and territory size.
The relief of Bashkortostan is extremely diverse. The highest point in the region is the Zigalga ridge (1427 m). Plains and hills are well suited for agriculture, so the population of Bashkiria has long been engaged in cattle breeding and crop production. The republic is rich in water resources; the basins of such rivers as the Volga, Ural and Ob are located here. 12 thousand rivers of various sizes flow through the territory of Bashkiria; there are 2,700 lakes, mostly of spring origin. Also, 440 artificial reservoirs have been created here.
The region has large mineral reserves. Thus, deposits of oil, gold, iron ore, copper, natural gas, and zinc have been discovered here. Bashkiria is located in the temperate zone; there are many mixed forests, forest-steppes and steppes on its territory. There are three large reserves and several nature reserves. Bashkortostan borders on such subjects of the Federation as the Sverdlovsk, Chelyabinsk and Orenburg regions, Udmurtia and Tatarstan.