Before you understand how you can lubricate the battery terminals, you should understand the question: why lubricate them. And they lubricate the battery terminals of cars so that a white coating (oxide) does not form on them. The oxidation itself occurs from electrolyte vapors and under the influence of other aggressive environments, which include air (it contains oxygen). The oxidation process is invisible at first, but negatively affects the operation of the battery. So much so that it can begin to quickly discharge (due to current leakage), there will be a problem with starting the engine, and then you will have to completely restore the terminals. Want to avoid this?
Is it possible to lubricate battery terminals?
The main question that novice car owners ask is whether it is possible to coat the battery terminals with lubricant. The answer is unequivocal - “Yes”.
But this is not done for better contact, as many believe, but in order to protect the metal from oxidation.
The same Litol or other processing agents are dielectrics, therefore they reduce the conductivity of contacts.
Their task is to protect non-ferrous metals from oxidative processes and the formation of dangerous deposits on the surface.
Where to lubricate, outside or inside
The lubricant should protect the battery terminals from oxidation while maintaining the transmission of current through the contact surfaces. Some copper-based products are applied to the pins before connecting the battery to the vehicle's on-board network.
Battery terminal lubrication
It is recommended to pre-clean the surface mechanically, install the wire terminal blocks, and then treat the surfaces with a protective lubricant. Some protective preparations contain dielectrics; getting the substance between contact surfaces impairs the conductivity of electric current.
What are the causes of oxidation?
The appearance of oxide on the battery terminals is a signal to the car owner about the need to take urgent action.
But you should not limit yourself to just cleaning and processing the leads - it is important to understand the reasons for the appearance of plaque.
These include:
- Poor contact between tip and battery
. The problem occurs when a gap occurs into which electrolyte vapors enter from the power source. To prevent the terminals from oxidizing in the future, it is necessary to ensure maximum tightness of the connections. - Damage to the terminal (scratches, chips, or others)
. In such a situation, the best solution is to replace the damaged element. - Violation of tightness in the area where the terminals exit from the battery
. If there is such a problem, electrolyte leaks from the power source, which ends up on the surface of the terminals. The solution is to ensure proper tightness. - Recharging the generator
. You should not delay with such a problem, because often the malfunction is caused by a malfunction of the voltage regulator. But each specific case must be considered individually. - There is no ground on the motor
. The solution is to ensure a high-quality connection between the wire and the housing.
The reasons for the appearance of plaque on terminals can be divided into two categories:
- Electrolyte leakage caused by the condition of the battery or problems with other components.
- Failure of electrical network elements.
General reason or a little theory
Self-discharge and oxidation of contacts,
turns out to be interconnected. The reason for these phenomena is the combination of water, dirt (dust) and sulfuric acid from the battery itself on the surface of the battery and its contacts.
Sulfuric acid
- this is an integral part of the electrolyte, it tends to slowly evaporate, this process is called electrolyte leakage (especially when the battery almost boils in the summer under the hood of a hot car), and can also leak where the contacts exit the housing. Sulfuric acid vapor loves water and immediately combines with it, forming a solution of sulfuric acid. Let's remember this.
Dust, dirt, moisture
- they are always under the hood and over time they cover any surface there with a durable coating, including the battery and its contacts. Water holds dust, dust holds water - in general, there is always water in this coating. It dissolves some of the dust, and then in the area of the battery, sulfuric acid is added to it - and this is no longer water, which in its pure form does not conduct electric current, but a conductive solution, an electrolyte, almost the same as in the battery.
It is not for nothing that experienced craftsmen never install or remove a used, not new, battery without protective clothing and gloves. It seems like I didn’t touch the battery with my pants, but lo and behold, a few days later, out of nowhere, a hole appeared. The acid ate away, slowly but surely.
What is the danger of terminal oxidation?
Every car owner must monitor the condition of the battery and take measures to eliminate deposits.
If you do nothing, the following problems are possible:
- Difficulty starting the engine. This malfunction is typical for cars of domestic brands. Most often, the “positive” terminal suffers, which turns out to be contaminated.
- Decrease (dip) in on-board network voltage during engine start-up. When you turn the ignition key, the starter consumes more than 100 Amperes of current, so even insignificant resistance creates problems and “pulls” the voltage onto itself.
- The effect of self-discharge of the battery occurs. The reason is the appearance on the surface of the product of an oxide film capable of conducting electric current.
The driver’s task is to monitor the condition of the battery, to do everything possible to ensure that the connection points of the battery clamps and terminals do not oxidize.
Ignoring the problem will inevitably lead to the inability to start the engine due to self-discharge or other problems mentioned above.
Timely processing of the terminals using high-quality lubricant guarantees protection against oxides. But more on that below.
Bad battery or poor contact with the battery?
Really, why won't the car start? The most common reason is the battery. The car does not have enough voltage-current-charge, the starter clicks, but the car does not start. Battery, right? Yes, but not really. There are nuances:
1. The battery is really dead.
A kind person with a cigarette lighter cord will help you start the car, or you will have to connect the charger for 15 minutes, or a newfangled capacitor device found in your neighbor’s garage will help. We set off, but the question remained: “Why did the battery die?” There are two options here:
- Maybe the battery has really expired, it doesn’t hold voltage, has a high self-discharge, etc. Charging doesn't help for long.
- The battery has discharged something, and after charging it, the car starts with a bang. We need to look for the reason. Besides the banal ones (the lights were on all night), there is one reason that even experienced drivers sometimes forget about: self-discharge directly into the dirty body. We will return to this reason shortly.
We recommend reading the article on how to extend the life of your battery.
2. The battery has a decent charge, but there is not enough current.
This means there is poor contact in the battery terminals, where the wires to the body and the starter are attached to it. And here are two options:
- The clamp on one or both terminals of the battery is simply not tightened, it dangles, and is easily turned by hand. Turn it a little in one direction or the other, pressing down so that it sits deeper, tighten the bolt and - go ahead!
- The contacts have oxidized, or even become completely covered with an unsightly layer of oxides. Oxides do not conduct electric current well, and their layer can greatly increase the resistance of the starter power circuit, making the normal starting current unattainable. To leave, clean the terminal and clamp with improvised means (knife, rag), attach the clamp, slightly twisting it (as in the previous case) and tighten it. The car should start.
But remember, this is only a temporary measure, and the battery persistently requires your attention.
There is a video waiting for you at the very end of the article!
How to deal with oxidation? Basic methods
Experienced car owners recommend several ways to combat the appearance of plaque on the surface of battery terminals.
The main ones include:
Cleaning with sandpaper.
This method is considered one of the simplest and most accessible. To implement it, it is worth preparing sandpaper or a special brush with metal bristles.
The first step is to remove the terminal from the electrode.
The next step is to take “fine” sandpaper or a brush with metal bristles, and then process the junction of the battery terminal and the tip.
Special attention should be paid to the inside of the terminal. In this case, the work is carried out until shine appears.
The main thing here is not to overdo it, so as not to damage the surface of the product.
Petrol.
The second option is to use gasoline, which also copes well with plaque.
To process it, just moisten a small piece of cloth, then wipe the battery terminals and electrodes with a rag until the surface is completely clear of plaque.
Caution should be exercised during processing due to the easy flammability of gasoline. In addition, it is not recommended to let the device come into contact with rubber or plastic elements (gasoline has a negative effect on them and can destroy them).
The main method of combating oxidation is to monitor the condition of the battery and protect it from electrolyte leakage through the electrodes.
If such a problem occurs, the car owner has two options:
Replace the faulty unit. This option is the most effective, but expensive. Not all car owners are ready to immediately spend a large sum on purchasing a new battery. But if a break appears at the electrode output, there is no other way.
Provide high-quality insulation. Here you can use one of the already proven methods or use modern means of protection (more on them below).
Application process
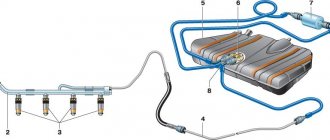
An important point is mechanical cleansing of the treated areas (see above). In addition, you need to ensure the integrity of the battery. You should also eliminate, if possible, the causes of the problem. For example, install a gas outlet pipe to remove aggressive vapors.
After cleansing, you can apply a thin layer of protective lubricant. It is possible to process the terminals themselves directly and then crimp them. But this method depends on the material used. After mechanical treatment, you don’t have to do this, but apply a protective agent directly on top of the joints.
Is a lubricant capable of removing oxides from the metal surface?
There is an opinion among beginners that a good lubricant protects the surface and removes existing traces of oxidation. This is mistake.
If oxides appear on the terminal, applying a special compound (even the most expensive) will do nothing.
The only solution is to remove the tips and clean the terminals until shiny, and after connection they are treated with a special lubricant.
The task of such lubricants is not to eliminate the consequences, but to prevent their occurrence.
What do oxide films lead to?
The formed oxide film does not provide the contact area required by the vehicle design, which does not allow the required current to be supplied to the starter. For example, when starting a cold engine in a passenger car, the current reaches values of more than 200 A. If the contact zone is oxidized, then the current passed will not be enough to crank the engine crankshaft.
An additional problem with terminal oxidation is local overheating of the contact pads at the time of start-up. The passing current of high strength heats the area to the melting point of lead alloys, which leads to the destruction of the battery and electrical wiring of the car.
Oxide films and electrolyte drips form paths connecting the battery poles and accelerating the self-discharge process.
As a result, the current source loses capacity; with deep discharge, degradation of the active mass and plates begins, leading to irreversible damage to the battery.
Secrets of prevention
It is important to understand that even in a new battery, minor acid vapors occur. This means that the best way to protect against trouble is to ensure the product is sealed.
It is prohibited to apply special materials directly to the contact site. First, the area is cleaned, wiped, and after connection the treatment is carried out.
Popular methods of protection include:
- Felt and oil
. This technique has been known for many decades. The main advantage is accessibility, which determines the popularity of the products among car owners. To avoid electrolyte vapors from entering the terminal and the appearance of oxides, it is enough to cover it with felt (pre-treated with machine oil). All that is required is to cut a round felt gasket with a hole in the center, then soak it in oil and put it on the battery terminal. After this, the on-board network terminal is put on the contact. It must be screwed on, and a felt pad soaked in oil must be placed on top. - Classic remedies are Vaseline, grease and varnish
. Experts assure that to protect the terminals you can use almost any liquid composition that provides insulation and is resistant to shampoos. Silicone-based lubricants are suitable here, they are affordable and do not absorb various debris (dust and dirt). - Anti-fat
- a special lubricating composition that has anti-corrosion properties and does not impair the conductivity of the contact connection. This product is sold in aerosol form, and processing is carried out by conventional application to the terminals. Anti-grease is sold in many automotive stores. - Felt washers
. Here the processing principle is the same as in the case of felt products. The washer is put on top to prevent the terminals from oxidizing.
Old-fashioned methods of lubrication of terminals
In those days, they didn’t sell a variety of beautiful tubes of auto chemicals. What was available, at hand - that’s how we dealt. These methods still work today.
- Felt and oil
- Indeed, from our grandfathers, the method has been known for a long time. A ring of the required dimensions is cut out of thin felt, soaked in machine oil and put on the cleaned terminal. The clamp is put on with a twist to increase the contact area. Sometimes the top of the knot was covered with an additional plate of oiled felt. The entire surface of the metal was lubricated with oil. - Consistent oil
- If liquid machine oil was used in the previous method, then the entire contact assembly was covered with thick oil - grease, lithol, nigrol, petroleum jelly, without felt. There is a certain difference of opinion here - some consider this method to be the best, while others accept it as a temporary measure until it becomes possible to use a modern means. Of course, this is not what oil is intended for. But if you clean and recoat the contacts twice a year, the method works. - Copper grease
- Both the grease is rare and the method is rare - but effective due to the special properties of copper grease. These are stability of characteristics, viscosity, antioxidant properties. - Wax (paraffin)
is an effective option; it is a good dielectric and also adheres well to the surface of terminals and clamps. - Silicone
is a material that can be given a variety of properties. As a terminal agent it has certain advantages. Silicone is resistant to aggressive environments and water, and can operate at temperatures from -60°C to +180°C. It is important that it is silicone grease without conductive additives.
Silicone coating requires constant monitoring and frequent processing.
How to lubricate the terminals to ensure contact and protect them from oxidation?
Let's consider what actions to take to prevent the terminals and wire tips from oxidizing.
Here it is worth highlighting several groups of products:
- "Old-fashioned" methods
. This implies the use of time-tested lubricants - Nigrol, Litol or grease. After cleaning the terminals and pulling them tightly, a lubricating compound is applied to the surface. With proper processing, reliable protection against breakdown and leakage for 6-8 months is provided. - Petrolatum
is another effective means of protecting against potential oxidation problems. The use of technical and pharmacy versions of the product is allowed. But many experts are skeptical about Vaseline (unlike grease). The reason is that it protects the metal surface from moisture and prevents the terminal from sticking to the battery terminal. The main disadvantage of Vaseline is its high resistance, which causes problems with conductivity. To avoid complications, it is recommended to add a graphite-based lubricant to the composition. - Machine oil
. This method of protection is intended for the laziest car owners who do not want to spend time looking for lubricant or money to buy it. Each time you check the oil level, apply a small amount of oil to the terminal (to prevent the terminals from oxidizing). If you do this work regularly, the battery will not need maintenance soon. - Solid oil
. This option is considered one of the simplest and most accessible. But many car owners make a mistake - they apply a lubricant between the wires and battery terminals. Subsequently, under the influence of high temperatures, the lubricating composition hardens and sets, and a dense and dry crust appears. Its peculiarity lies in the inability to conduct current, which increases the risk of loss of contact. In the future, cleaning off the hardened composition is a real problem. By the way, such difficulties can also be encountered when using other lubricating compounds (including modern ones).
Why do battery terminals oxidize?
To effectively deal with the problem of terminal oxidation, you need to know under what conditions this process develops especially rapidly.
The most common reasons are:
- Insufficient contact between lead pins and terminals
- Electrolyte release through microcracks
Electrolyte leakage often occurs when the battery is recharged. The cause of this problem is incorrect operation of the generator or circuit, too low density of the electrolyte, short circuit of the battery cells, damage to the battery case.
Another reason may be faults in the vehicle wiring, which arise as a result of a poor connection between the clamp and the battery contact.
When the openings of the battery “cans” become clogged with debris, the level of electrolyte pressure in the cans themselves increases. This causes the formation of cracks and crevices through which the electrolyte escapes.
In addition, oxidation may indicate that the battery has already exhausted its service life and may soon fail.
To reduce the intensity of the oxidation process, the terminals must be tightened well with a wrench. If this is not done, then very soon the car will stop starting due to the resulting “scale”, which will prevent the normal flow of electric current.
When the electrolyte reaches the surface, it accelerates the oxidation process, so thin felt rings can be placed on the lead pins.
Lead and its alloys, which form the basis of battery terminals and terminals at the ends of the on-board network conductors, react with oxygen in the air. As a result, oxide films are formed on surfaces. The high air temperature under the hood promotes intense reactions.
When moisture gets into the gaps between the contacts, electrolysis begins, the products of which activate oxidation processes. This phenomenon is especially noticeable on older cars that use copper terminals. The lead of the battery terminals and copper form an electric pair, creating an EMF, under the influence of which electrolysis proceeds more intensely.
In the event of leakage of electrolyte and its vapors from the battery (if the integrity of the case is violated, the caps on the banks of the battery being serviced are not tightly closed, the terminals are loosely sealed or they become loose due to constant vibrations), exposure to sulfuric acid leads to the formation of sulfates on the surfaces and in the contact gaps.
What are the disadvantages of using old Litol-type lubricants?
The main advantage of the “old-fashioned” terminal processing methods is accessibility.
At the same time, budget lubricants have a number of disadvantages:
The presence of a loose (“loose”) structure, which promotes the adhesion of dirt and low resistance to shampoo (easily washed off).
Lack of useful additives and additives. There is also no dye provided, which is a serious disadvantage for many car owners.
Insufficient energy conductivity, which reduces the efficiency of the power source.
Despite a number of shortcomings, many car owners continue to use Litol-24 lubricants.
What products are used for protection and treatment?
The main purpose of lubricants is to protect contacts and elements that are made of lead. It doesn’t hurt to understand why battery terminals oxidize and require reliable protection.
Reasons for frequent oxidation:
- lead is extremely susceptible to such effects;
- aggressive environmental influences aggravate the situation;
- Leaks regularly occur on serviced models;
- older cars were made with copper terminals, but the contacts are made of lead, as a result oxidation occurs faster.
Among other useful functions of lubricants, improvement of electrical contact stands out. Modern products are distinguished by such properties. When thinking about how to lubricate battery terminals for better contact, you should pay attention to the latest developments. They differ in characteristics; they paint blurred areas in a certain shade - blue, red.
Popular formulations:
- Liqui Moly is a famous manufacturer that produces reliable lubricant, similar to technical Vaseline.
- Molykote HSC Plus is a product suitable for different drives. It is characterized by high electrical conductivity and the ability to operate under significant temperature fluctuations.
- VMPAuto MC1710 is a substance that helps maintain stable voltage. Easy to apply.
- Cyatim is a domestic product that attracts with the most affordable price. Its properties are similar to Litol. Therefore, conductivity is insufficient.
Traditional methods are presented:
- felt pads pre-soaked in oil;
- apply grease, technical petroleum jelly with graphite lubricant;
- washers, felt gaskets;
This provides excellent protection, including from moisture, and eliminates soldering of terminals. Problems can arise at very high temperatures when the solid oil hardens. Therefore, it is preferable to use modern means, aerosols.
Subtleties of care and processing
Timely care and selection of appropriate materials allows you to preserve the battery life and its properties. Using cleaning products will help fight oxides if they already exist. They must be removed, otherwise the protective equipment will be useless.
The cleaning process includes several stages:
- The power plant is switched off, which prevents a short circuit. It is important to remove all metal jewelry from your hands.
- Depending on the outputs, the required key is selected.
- Work begins by loosening the nuts, which allows you to remove the terminals. It is important to remember how to disconnect the battery terminals - work begins with the negative terminal to avoid short circuiting.
- After disconnecting the positive terminal, the battery is inspected for damage. And if there are signs of wear on the wires and elements, replacement will be required.
- A soda solution allows you to remove traces of electrolyte vapors and oxides. To do this, dilute a teaspoon of soda in a 250 ml cup of water.
- Using a brush (tooth or wire) you can easily remove unwanted deposits.
- After complete cleaning, the terminals and the drive are washed with water. It is noteworthy that on a clean device, oxides appear more slowly.
The final step is to connect the battery terminals and process the cleaned areas. When connecting, it is worth remembering that the positive terminals are connected first.
Emergency care
If you don’t have time for thorough treatment, you should use emergency cleaning products. They differ in their mode of action and characteristics. Therefore, it is important to study the instructions and not forget about protective equipment.
If you don’t have any tools on hand to carry out the work, you can use regular cola. You will need to prepare protective gloves and select a wrench.
Main stages:
- The terminals become loose and cannot be removed.
- Cola flows from the center of the battery to one and then to the other edge.
- After 2 minutes, the device and contaminated areas are washed with water.
- The terminals are tightened.
When treating connections with lubricant, there is no need to coat the terminals and contacts separately. Protection is applied after connection.
Options for self-recovery of terminals
Timely maintenance of the drive, its elements, connections, removal of contaminants and oxides allows you to maintain good contact. Otherwise, it cannot be ruled out that the terminals will melt. More often this happens in the summer. There are several options to solve the problem. You can replace the terminals or think about how to restore the terminal on the battery yourself.
Methods for self-recovery:
- A AA battery is made into a mold by melting lead and tin and the cell is restored. This solution is only suitable for partially melted elements. Before performing the procedure, the fusion site is cleaned and a mold is put on.
- Lead fusion is used if the terminal is completely destroyed. Take the core and shell of the battery. The method is suitable for recovery on the road.
- If an element breaks off, restoration can be carried out using self-tapping screws. Take pieces of equal size and drill holes in them, no more than 5 mm in diameter. Next, use a conductive lubricant necessary for processing the planes, connect them with a screw or self-tapping screw.
Correct operation, cleaning, and care of the battery helps to avoid unpleasant incidents.
As you know, battery cells and parts operate in an aggressive chemical environment. Therefore, for a car enthusiast, the question of how to lubricate the battery terminals so that they do not oxidize always remains relevant.
What lubricants are suitable for winter?
To protect the terminals from oxidation in cold weather, the use of special compounds is recommended.
The most popular ones include:
- Molykote HSC Plus
- a composition that is intended for FIAMM series batteries, but can also be used for other devices. The advantages are high electrical conductivity and the ability to operate in a wide temperature range (-30...+1100 degrees Celsius). - Cyatim
is an affordable option. Its main disadvantage is low conductivity. - Spray cleaners
. In this series, products from the following manufacturers are in demand - Hi-Gear, SVITOL and others. It is based on purified oil, which, once it hits the surface, “squeezes out” moisture and provides a reliable protective film layer. The latter protects the surface from oxidative processes and the appearance of dirt. The main disadvantage is the short-term effect of the composition. - Grease-type lubricants (options: LIQUI MOLY, Gunk)
. In structure, these are thick compositions that have the appearance of a cream and also have an oil base. Their advantage lies in the ease of application, because you don’t have to worry about getting the composition where it’s not needed. In addition, the lubricant stays on the surface longer and cannot be washed off with shampoo. That is why many craftsmen recommend choosing just such products for winter processing.
People's rating of the best remedies
Among the huge number of products on the market, we will highlight the most popular, time-tested protective lubricants for terminals.
HSC Plus from Molykote
Electrically conductive lubricant based on mineral oil with a thickener, containing metal powder. Works in a wide temperature range. Available in paste and aerosol form.
Ciatim 201
Universal instrument lithium grease, frost-resistant and refractory, inexpensive.
Batterie-Pol-Fett from LIQUI MOLY
Special lubricant for battery terminals. Synthetic acid-resistant Vaseline, red in color. Completely blocks the access of moisture to the contact area. Reduces contact resistance.
Battery Pole Spray by Berner
Specialized aerosol for battery terminals. Improves contact. Well protects against corrosion.
MC 1710 from VMPAVTO company
Lubricating paste for terminals. Apply only to the dressed terminal. Protects against corrosion (contains additives), creates a protective film.
BATTERIE-POL-SCHUTZ from Presto
Protective aerosol. Does not allow sliding discharges, protects against corrosion. Blue color.
Kupfer-Spray from LIQUI MOLY
Universal copper spray for high temperature connections, also suitable for terminals.
How to apply lubricant, and where is it best to apply it?
To extend the life of the lubricant and protect the terminals from oxides, it is important to strictly follow the technology for applying protective compounds.
Here the algorithm of actions is as follows:
- Cleaning the surface of the terminal and nearby areas from contamination. This is required to reduce resistance to a minimum.
- Cleaning the terminals and putting them on the battery. Next, it remains to tighten the nut for maximum fixation.
- Treatment of the top and side of the tip and outlet. It is important not to miss anything here to avoid moisture getting on the surface.
Please note that it is recommended to process the entire node, and not just some specific elements.
It is worth remembering that lubricating the inside of the terminal before applying it to the battery is prohibited. Otherwise, it will not be possible to achieve high-quality contact, and the treatment itself will only cause harm.
Precautionary measures
As a rule, all technical lubricants are toxic to the human body to one degree or another. Therefore, work on their application must be carried out with gloves. If technical lubricants come into contact with your skin, wash them off immediately with warm water and soap.
Important! When applying protective lubricant, you need to make sure that it does not get on rubber tubes, gaskets and other surfaces that do not need such treatment. This is necessary in order to avoid the destructive effects of the components contained in lubricants on these materials.
Is it possible to clean the terminals mechanically, for example using sandpaper?
If you act without fanaticism, such processing is permitted. Moreover, many manufacturers recommend cleaning the contacts with fine-grit sandpaper before applying the protective compound.
This ensures that oxides are removed and the surface is cleaned of dirt. Previously, special “brushes” with metal bristles (external or internal type) were used for processing.
Litol
Since Soviet times, thick non-conductive lubricants have been used as the main means of protecting against corrosion:
Currently, lithol continues to be relevant for protecting battery terminals due to its greatest efficiency. This fuel and lubricant has good penetrating properties, covering the area of metal surfaces of the contact zone with an insulating layer.
With natural wear and temperature changes, the physical state and qualities of lithol change and it hardens. Therefore, during the implementation of routine measures for cleaning the contacts (which are required twice a year), the remnants of the old lubricant are completely removed, after which a fresh one is applied.
Why lubricate?
How to properly charge a gel battery? 2 ways: regular and special charger
There are several reasons here, but initially this is one reason, so:
- In order to improve the electrical contact of the circuit, there are lubricants that actually improve the transmission properties of electricity
- Perhaps the most important reason is its PROTECTIVE PROPERTIES. The thing is that lead (terminals are also made from it) tends to oxidize. Especially under the influence of the environment. Another disadvantage is that on old cars the terminals were made of copper, and the contacts were made of lead; various metals developed oxidation over time. Well, if you take old batteries that were serviced (made using antipyretic technology), then their electrolyte often boiled out of the cans, and quite violently. The fumes came along with some of the sulfuric acid, which could also get on the contacts. Here you go - OXIDATION AGAIN.
By the way, many auto electricians recommend treating the mass of the body with such protective compounds; it can also oxidize over time. Actually, so that nothing oxidizes these contacts and lubricates the terminals, if this is not 100% protection, then at least decay products do not appear for quite a long time.
Silicone Grease
A product with excellent characteristics. For processing, it is better to purchase composition options without conductive additives. The lubricant is valued because it not only creates a protective barrier at the molecular level, but also repels aggressive substances. However, this product is only suitable for complex use together with other anti-corrosion drugs. They are first applied to the surfaces, after which the devices are connected.
The second disadvantage of the lubricant is its increased fluidity, which causes additional trouble for the performer of the operation. Therefore, it should be systematically added, then carefully removed the excess amount of material that does not fall into the contact zone.