Motorists quite often encounter situations when the battery is severely discharged and its charge is no longer enough to start the engine.
Usually in such situations there is only one way out. This means removing the battery, putting it on charge, and then returning to the usual operating mode.
But it also happens that when the battery is discharged, it is no longer possible to restore it. The battery does not react in any way when connected to a charger, and when started from a ROM or booster, the generator does not provide charging.
Here you need to know what deep discharge is, why it is dangerous, and how to resuscitate the battery.
Design and principle of operation of the battery
The body of the product is made of propylene; this material was chosen for two main reasons:
- Does not conduct current;
- Not destroyed by acid
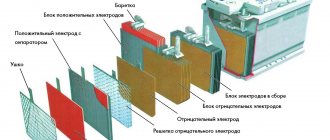
One device includes six interconnected batteries. A separate battery combines negative and positive electrodes (a lead alloy is used for their manufacture, a lead-calcium composition is used for negative electrodes) filled with active mass.
Insulation of layers of opposite charge is ensured by a plastic separator. In order to improve corrosion resistance, the lead-calcium alloy for electrodes can be diluted with silver or tin.
The active mass of negative electrodes consists of sponge lead, positive - of lead dioxide.
There are two types of batteries:
- With liquid electrolyte.
- With a special material pre-impregnated with non-liquid electrolyte.
Today, the most common batteries are those with liquid electrolyte.
The operating principle is based on the conversion of electrical energy into chemical energy during charging; during discharge, the opposite effect occurs - chemical energy is converted into electrical energy.
Battery discharge occurs as a result of connecting consumers: the active mass of the electrodes (negative and positive) interacts with the electrolyte.
As a result, lead sulfate is generated with water and the density level of the electrolyte drops. When the generator is working properly, it charges the battery while the engine is running.
The battery can also be charged with a special device; as a result of the charge, lead sulfate and water are converted into lead, lead dioxide and sulfuric acid, thus increasing the density level.
Note! The charge must be carried out taking into account the recommended electrical voltage; if this operating rule is violated, the service life of the device may become significantly less than specified.
As a result of high voltage, the electrolyte level drops; low voltage can cause the battery to not be fully charged. In general, the battery life is about five years, it all depends on the conditions in which the device is used.
Device parameters:
- Nominal capacity. This indicator is measured in ampere-hours (Ah), depending on the energy output of the charged device during discharge (20 hours). For example, a device with a capacity of 50 Ah delivers a current of 2.5 A for twenty hours.
- The nominal voltage consists of the voltage of the individual batteries; for a passenger car it is 12 V.
- The cold cranking current indicator indicates the vehicle’s ability to start during cold periods. The higher the indicator, the easier it is for the engine to start in cold weather.
The main reasons for battery failure
Most likely, every motorist is familiar with the design of the battery. But, nevertheless, we will briefly refresh this knowledge.
A battery is a device that, on the one hand, converts the energy obtained as a result of a chemical reaction into electric current, and on the other hand, is capable of collecting (accumulating) the electric current produced by the car's generator. To ensure these processes, the battery contains two sets of lead plates with positive and negative charges, and an alkaline solution, which serves as the so-called electrolyte. These are actually all the components of such a complex device at first glance. So what problems can happen to it?
- Destruction of plates. The reasons for this may be factors such as the use of an electrolyte that is not suitable for climatic conditions, “overcharging” of the battery, a long discharged state, mechanical damage due to poor fastening of the battery.
- Short circuit. The reasons in this case are the destruction of the separators or the formation of growths between the plates.
- Sulfation. Perhaps the most unpleasant problem possible. Occurs when a discharged battery is stored for a long time or there is a lack of electrolyte during operation.
- Electrolyte contamination. A common “disease” of the batteries being discussed. It arises quite simply and is just as easy to fix with your own hands.
- Intensive self-discharge. One of the most common malfunctions. There can be many reasons for this – from a poorly tensioned alternator belt to a low level of acid in the battery.
- Reversing the polarity of batteries. The cause of this problem may be incorrect and prolonged switching on of the battery. Eliminated by several discharge-charge cycles.
So, having dealt with the main “diseases”, it’s time to talk about how to cure them. Simply put, let's move on to practical recommendations that will help you update a battery that has become unusable.
When recovery is not possible
If the electrolyte inside turns out to be black or brownish-brown, it is most likely impossible to restore the battery. If the battery is swollen and swollen, it should be disposed of immediately.
The plates may crumble or fall apart altogether, as a result of which one or more “cans” may short-circuit in the battery. After a short circuit, restoring a car battery is also not recommended. Therefore, you should always look at what happened inside the battery before restoring it - of course, observing possible safety precautions.
To avoid letting the power source reach this state, follow the rules for using batteries.
What batteries can be restored?
Let us immediately determine that not all lead-acid batteries can be restored. For example, gel (GE) batteries and batteries made using AGM technology cannot be restored. There are, of course, craftsmen who refill these types of batteries with dried electrolyte with distilled water, but after such restoration the batteries are only suitable for emergency power supply at the dacha - listen to the radio and light a 12-volt light bulb.
This type of battery is practically not susceptible to sulfation, although if you try really hard, you can sulfate it. For such a battery, there is only one way - recycling. Therefore, further discussion will only be about lead-acid batteries with liquid electrolyte.
You can try to revive the battery if it does not hold capacity. That is, it charges quickly and discharges just as quickly. Moreover, upon completion of charging, a seemingly normal voltage value is established at the battery terminals - 12.66 V (measured 10 minutes after removing the battery from charging).
If one of the battery cells short-circuits (usually occurs when the plates fall off), then, most likely, it will no longer be possible to revive such a device. A characteristic sign of an internal short circuit is that the voltage of a fully charged battery is 10.6 V (one bank is shorted) or lower.
Healthy! If the plates are slightly shed, you can try to eliminate the short circuit by thoroughly and intensively washing the battery with distilled water. This will help wash away the crumbled elements from the bottom that caused the short circuit.
A burst battery case will most likely not be repaired properly either. The crack, of course, can be sealed or soldered - there are no problems. You can use a hot glue gun or a regular soldering iron. But a car battery is operated under very harsh conditions of vibration and shaking, so sooner or later this seam will burst anyway, flooding the engine compartment with acid. Moreover, it will burst at the most inopportune moment. A battery with leaked electrolyte is no longer a battery. It’s not just that it won’t start the engine, it won’t light the light bulb. Therefore, it is better to refuse repairs immediately. Unless you use this battery at home for backup power.
It will not be possible to restore a frozen car battery either. This usually happens when parked for a long time in severe frost, if the battery is partially discharged or the electrolyte density is not selected for the season. And, of course, it will not be possible to revive a battery with an internal break. There will be no voltage at the battery terminals at all, and nothing can be done about it. Unless you drain the acid from the damaged jar, rinse it with plain water and close it by sticking nails between the plates. A better option is to open the damaged can and close it properly. The voltage across the entire battery will drop to 10.6 V, but it will work properly. If the electricity is turned off at the dacha, we will not be left without light and can use it to power the laptop through a boost converter.
Most of the methods proposed in this article involve working with electrolyte, which will have to be added, drained, or refilled. Therefore, they are suitable for serviced battery models. But it is quite possible to use them on a maintenance-free battery. To do this, it is enough to drill holes in each can in places where there were previously technological holes for factory filling. Subsequently, they can be closed with stoppers from medical vials.
Why you shouldn’t let your battery reach a deep discharge state
Battery discharge is a completely natural and normal phenomenon. After all, batteries are designed to accumulate energy, release it, and then accumulate it again. And so it goes cyclically. That is, batteries are multi-chargers. There is no need to change the battery every time it loses its charge. After all, she makes up for it.
But the design of modern batteries is far from perfect. It has a number of problems and requirements:
- Recharging is not allowed, as this provokes shedding of the plates;
- It is extremely undesirable to let the battery become deeply discharged;
- It is always important to maintain the correct electrolyte density;
- the working fluid must be at a stable level;
- avoid shorting cans, etc.
How long the battery can last if a car battery is deeply discharged largely depends on the battery itself, its current condition and the efficiency of resuscitation actions.
Before you find out what to do in such a situation, it is necessary to clarify the reason for such a high danger of a deep (complete) discharge of the starter battery.
Acid batteries contain an electrolyte that has a certain density. The electrolyte is presented in the form of a mixture of sulfuric acid and distilled water.
As the battery discharges, acid gradually begins to settle on the positive lead plates in the form of salt. And the stronger the discharge, the more active and voluminous these deposits are. The density drops, significantly different from the norm.
The optimal density indicator is considered to be 1.27 g/cm³.
A deep discharge can be described as the minimum threshold for battery discharge, below which there is simply nowhere to go. If the battery is set to zero, a chemical process takes place inside, stimulating the deposition of salts on surfaces. To remove deposits, you must connect the battery to a charger as soon as possible. Or let it start charging from the car's generator.
Thus, the density is normalized, the salt crystals are destroyed, and the battery’s performance is restored.
It would seem that with a deep discharge, you can simply connect the battery to the charger, and everything will return to normal. This is a common misconception.
At zero charge, the density of the salts increases so much that upon subsequent charging they are no longer destroyed, but are firmly deposited on the surfaces of the plates.
That is, the lead plate is almost completely covered with a solid salt layer. And since the battery is charged due to the interaction of lead and electrolyte, in such a situation the battery will no longer be charged.
Such a battery is no longer capable of accumulating a charge.
With each deep discharge, the battery loses 2-3% of its capacity, which can no longer be restored.
Because of this, when the battery experiences about 10 full discharges, it is no longer possible to count on 30% capacity. With such losses, the accumulated charge is not enough to start the engine.
A discharge of up to 10.5–11 V is considered deep. It is this threshold that is considered critical when the sulfation process actively begins to occur. That is, a precipitate in the form of salt crystals begins to appear.
Effective ways to restore a car battery
Knowing how to restore a car battery on your own can help you get rid of many problems.
Low current
I must say right away that this method is only relevant for acid-base batteries. I have not tried to restore gel ones with low current. And I don’t advise you, because I can’t say anything about the results.
- The structure of an acid-base battery includes lead negative and positive plates placed in sulfuric acid. As a child, I saw this lead element in the yard at every turn. Eh, there were times;
- The basis for bringing the device back to life is repeated charging;
- A prerequisite is the use of low current;
- You will need a UPS and a battery charger;
- From the uninterruptible power supply, the battery will be able to receive current with constant strength and characteristics, which will ensure competent sequential recovery;
- There is a mandatory break between procedures;
- The first charging is done at a low current, and during subsequent recharging processes it is gradually increased;
- As a result, your battery should stop charging;
- Include charging intermittently. Due to this, the potentials of the electrodes are equalized;
- Don't be afraid, you won't cause any harm to the electrodes using this method;
- Pauses are needed for the dense electrolyte to move from the plates to the interelectrode space;
- This technique promotes a gradual increase in the density parameters of the battery electrolyte;
- Wait until the voltage is 2.5 W and the density reaches the nominal value for your battery;
- Don't forget to turn it off periodically. In total, the process should be divided into 8 stages;
- When charging, it uses a current that is 10 times less than the capacity of a charged battery.
Not that difficult, but long. We'll have to be patient.
New electrolyte
If you don't want to wait a long time, try the next method. It involves replacing the old electrolyte with a new one. Practice has shown that the method is quite effective. When I tried it, I was skeptical. But no, everything was great.
- If necessary, unscrew the battery using a screwdriver and drain all liquid from the system;
- Rinse the structure using hot or warm water;
- Add 3 teaspoons of soda to 100 grams of water. Even the distillate is better;
- The solution is brought to a boil and poured in instead of the electrolyte. Wait 30 minutes and then drain. Carry out a similar procedure 3 more times;
- After the last addition of soda, rinse the device again several times with hot water so that all remaining alkali comes out;
- New electrolyte is poured inside and closed tightly.
This method is used on many types of car batteries. Then you still have to charge it for 24 hours. And then another 6 hours of charging every day for 10 days. Set charging in a mode between 14 and 16 W, and the current is no more than 10 A.
Repeated charging method
When it comes to how to restore an old car battery, this procedure is worth trying. Its main feature is the use of a low nominal charging current.
The essence of the method is that the memory should work intermittently. They are needed so that the potential difference on pairs of electrodes, which are lead plates, has time to level out. When using low currents, this procedure is safe and not considered destructive. The main effect is achieved by redirecting high-density electrolyte from the plates into the free space between the electrodes.
If poor battery performance is due to density variations in the banks, this method will be very effective.
During the charging process, the car owner must ensure that the voltage in each jar reaches 2.4 V, and the density of the sulfuric acid solution is equal to the nominal value. The main thing is to remember that the car battery needs to be given a break, during which the electrolyte is redistributed. The minimum that will allow you to reanimate the battery in this way is 8 charge-rest cycles.
Reverse charging
Many experts consider this procedure to be quite risky, since charging is carried out by connecting the battery in the wrong sequence - not plus to plus, as usual, but plus to minus. First you need to completely discharge the battery. You will also need a more powerful charger, capable of providing a current of 80 A and a voltage of at least 20 V.
During this procedure, intense boiling of the electrolyte will be observed - this cannot be avoided, so do not be alarmed. The charging time in this mode is about half an hour, after which it is recommended to drain the electrolyte, wash the battery, add fresh liquid and charge the battery using a conventional charger with a current of no more than 12 A.
Which batteries are not afraid of deep discharge?
Currently, we can distinguish car batteries that really are not afraid of a possible deep discharge. If we talk about what exactly these “fearless” batteries are, then attention is focused on GEL and AGM technologies.
It is in their case that the loss of charge will not be critical, and after charging the batteries they will be able to function normally for many more years.
These batteries are not afraid of discharge, since the electrolyte is not used in a liquid aggregate state, but in the form of a gel (GEL), or in the form of a liquid sealed in fiberglass mats.
It is because of this that salts practically cannot settle on the surfaces of the plates. But even here it was not possible to completely get rid of possible sulfation. It’s just that the number of charge-discharge cycles in which sulfation actually makes itself felt has been increased several times.
Chemical washing method
This procedure is used to eliminate sulfation and quickly restore a serviced battery at home. You will need 2 commercially available reagents - Trilon B and an ammonia solution. The procedure is as follows:
- Try to fully charge the battery, then drain all the electrolyte.
- Rinse the battery with distilled water.
- Prepare a solution by adding 5% ammonia and 2% Trilon B (based on the volume of water) to the distillate.
- Carefully pour the solution into the jars - a violent reaction will begin, accompanied by boiling and splashing.
- When the liquid stops boiling, drain it and rinse the battery again.
After washing, fill in the electrolyte and charge the battery again to the end. Flushing removes the excess layer of lead sulfate, so the battery capacity should be restored.
The final method for removing lead sulfate is to replace the electrolytic fluid with distilled water and charge for a long time at 14 volts. At the first stage, the battery is brought to a boil, then the voltage is reduced. The goal is to slowly dissolve the sulfate with water. At the second stage, the distillate is changed, and the voltage and charging current are set to a minimum. The operation is considered successful if the density of the solution does not drop within 2–3 days. The duration of the procedure may take 3–4 weeks.
Overview of the device
Lead acid batteries were invented more than 150 years ago. Since its invention, the design has remained virtually unchanged. The box contains lead plates (minus) and lead dioxide (plus). A dielectric is poured between them. It prevents the plates from shorting.
Packs of plates are placed in electrolyte. As a rule, there are 6 packs (cans) in a standard 12-volt battery. Each produces a voltage of about 2.1 Volts.
The battery is quite durable and resistant to mechanical damage. But it has a weak point - the electrolyte. Due to sulfuric acid, when deeply discharged, the battery may become inoperable. But the second component of the electrolyte, distilled water, has a negative effect on the battery when recharging.
Checking the battery for short circuit
Now let’s talk specifically about how you can identify a closed cell in a battery. The easiest way is to contact specialists. They will take the battery for testing for a certain fee and render a verdict over a period of time.
But it is quite possible to check the battery for a possible short circuit on your own, using practically available tools and a standard set of tools available to every car enthusiast. There are several ways to check yourself whether the battery is shorted. Each option has its own characteristics and difficulties in terms of independent implementation:
- Technical method. Simple, but at the same time very effective. The bottom line is to check the starter battery for a short using a multimeter. Old batteries even made it possible to measure the voltage on each bank. The design of modern batteries does not allow this. Therefore, it remains to make general voltage measurements. This is done upon completion of the charging procedure. If the multimeter shows 10-11 V, one of the cans is probably shorted.
- Visual method. When the battery is fully charged, but the charging voltage continues to flow to it, the process of electrolyte boiling begins. That is, gas is released and bubbles are formed. If 5 cans are boiling, and one is “silent,” it means that it is the problematic section of the battery being tested.
- Diagnosis by color. This test option is mainly suitable for serviceable batteries. After all, you can unscrew the caps and look into all the jars, thereby determining what color the electrolyte is there. A short circuit is indicated by a dark, black or cloudy color of the working fluid. Since modern batteries are mostly maintenance-free, you can only look at the condition of the electrolyte using the transparent part of the case. By holding the battery up to the light, you will potentially be able to see that the color of the electrolyte in one of the cans is different from the rest.
It also happens that the test shows the presence of a closed can. And here motorists have a natural question about what to do next.
It is potentially possible to restore the battery. But this is relevant mainly for specialists and in workshops where the appropriate equipment is available.
At the same time, car enthusiasts who like to rummage around in their cars have found a way to revive a battery with a closed bank with their own hands.
There is no guarantee that the presented recovery method will help. The motorist does this at his own peril and risk, accepting all responsibility.
Whether to engage in restoration or not is up to each individual.
How to revive a closed jar
There is no point in starting to restore the battery if several cells are short-circuited. The difficulty of performing this procedure on a maintenance-free battery can also make it wasteful compared to purchasing a new power source.
So, let's assume that you have already established the fact of a short circuit in the battery and know about the sad consequences of shorting the plates. We also wrote how to find a non-working jar - this can be done even without the presence of measuring instruments, by visually assessing the condition of the electrolyte.
So, what should you do if the battery bank is shorted, regardless of its type and design:
- disconnect the battery terminals and move it to a place convenient for work;
- unscrew the caps of each can;
- using a pear, drain all the electrolyte from the inoperative section;
- cut the plastic on the lid around the faulty can with a suitable tool;
- carefully disconnect the jumpers from adjacent battery segments;
- remove the package consisting of a set of plates and rinse it thoroughly;
- we subject it to a visual inspection in order to identify the source of the short circuit, for which we examine all the plates in turn for the presence of a defect that caused the short circuit;
- eliminate the cause of the short circuit;
- we wash the section to get rid of all the sediment accumulated on the bottom and walls;
- install the reanimated package in its original place;
- we perform the reverse operation of soldering the plates to connect the can with adjacent sections;
- fill the section with fresh electrolyte to the required level;
- We seal the cut part of the lid using adhesives or epoxy resin;
- tighten the plugs;
- put the battery on charge.
If the battery is not very old, the probability that it will be able to be charged to its usual values after such self-repair is quite high - at the level of 80–90%.
As for maintenance-free batteries, it should be remembered that they do not tolerate deep discharge - two such cases are enough for no restoration operations to bring the battery back to life.
How to tell if the battery bank is shorted
There is such a thing as car battery degradation. This means that over time, the declared battery capacity gradually decreases. The reasons may be different - from excessive sulfation of the plates to their shedding. This often results from a short circuit in one of the cans, as a result of which it becomes completely or partially inoperable.
But how to determine a short circuit on the battery?
Actually, the most accurate way is to measure the voltage on each of the plates. If the battery is collapsible, that is, it is possible to remove the cover, then this will not be difficult. But in most cases this is not possible, so it is quite problematic to understand that the problem is a short circuit.
The battery begins to spin poorly - measure the voltage at its terminals in a charged state. If it has dropped by an amount greater than the nominal voltage of one bank (this is 2.1 V), that is, it is in the range of 10.6–11.0 V, one can suspect that the culprit for such a drop in the most important battery parameter is a short circuit in one of the sections.
In addition to a decrease in voltage at the output terminals, a symptom of a short circuit in the battery cans can be a significant heating of its case - any short circuit is accompanied by similar thermal effects observed in the short circuit zone.
We will look at how to find out exactly whether the battery is shorted or not, below.
Let us only mention that the shorting of the plates occurs both as a result of direct contact (due to mechanical influences - impacts, shaking), and due to the accumulation of conductive sediments in the lower part of the can - this can be crumbling lead, scale or other pollutants.
Reanimation of a maintenance-free battery
Maintenance-free power supplies can also be restored. After all, it is prohibited to create a hole in the lid, as this will negatively affect the gas exhaust system.
The process of resuscitation of maintenance-free car batteries includes:
- Determination of the level of electrolytic composition. To do this, the body is illuminated with a lighting device.
- If there is a deficiency, a hole is prepared in the body. It should be slightly higher than the electrolytic composition level. The hole size is 2–4 mm.
- Filling with distilled water. This is done using a syringe.
- The hole can be closed using soldering.
The device's capacity is restored by discharging and charging. These processes are performed cyclically.
How to turn a maintenance-free battery into a serviceable one
The time comes and the car owner is no longer satisfied with the characteristics of the battery. If you have a sealed battery, you shouldn’t throw it away and buy a new one. You can make it serviceable if you open the lid using the method we described above. This will allow you to carry out a number of procedures necessary to maintain the functionality of the power source.
The ability to check and change the electrolyte level is one of the signs of battery serviceability. Closed batteries are equipped with an indicator that allows you to see the electrolyte level. Some containers are made of translucent materials or have markings that help you keep track of the amount of solution in all jars. The electrolyte level can be checked using a special tube or syringe by inserting them into the holes made in the lid. The normal amount of solution corresponds to 10–12 millimeters of liquid above the plates.
Do not forget that not only the acid level, but also its density is an important characteristic of battery performance. Data on the density of the solution can be obtained using a hydrometer. Take a syringe, insert it into the hole on the lid and take the electrolyte from the jar. Pour into the hydrometer and watch the readings. Too low a density (less than 1.22 g/cm3 at temperatures up to +6) does not allow the battery to work fully and indicates a small amount of electrolyte and the need to replenish it. Increased density or insufficient level of solution requires adding distilled water.
Battery hydrometer
Check the electrolyte level using a transparent tube.
Please note that excessive concentration of electrolyte leads to tragic consequences - sulfation and destruction of the plates.
The figure shows the electrolyte density values under various conditions.
After completing the work of adding water or acid solution, the holes in the lid must be sealed with sealant.
How to charge a maintenance-free car battery
So, if a classic battery can, subject to precautions, be charged with almost any direct current source (the simplest “old-fashioned” method is a light bulb with a diode connected in series, connected directly to the mains), then a maintenance-free battery requires a stabilized voltage source that has the ability to limit and control the charging current .
When charging a maintenance-free battery, the voltage should not exceed 14.5-14.8 V. A working battery in this case accepts a current determined by its internal resistance - being maximum at the beginning of the cycle, it gradually decreases. This is the basis of the operating principle of automatic chargers - when the current drops to a level of 200 milliamps, the charging cycle is interrupted.
If the charger exceeds the specified voltage, then in parallel with charging the battery, the decomposition of the water included in the electrolyte will begin. In the case of maintenance-free batteries, this is especially dangerous because it is impossible to see the “boiling”: in a classic battery, the process of gas formation is visible through the turned-out plugs, but here they either cannot be turned out or are covered with a battery cover.
Signs of a shorted car battery
As we have already noted, a short circuit is characterized by a redistribution of charge, and at the same time, abundant heat generation is observed at the point of short circuit. If you find that your battery is hot, this is one of the most common symptoms that there is a shorted battery.
However, this may also indicate a malfunction of the relay regulator - in this case, the battery will be subject to overcharging, which is also accompanied by increased heat generation. But in this case, it is not the plates that will heat up, but the electrolyte.
On serviced batteries, it is easiest to check whether the bank is closed while charging using a charger. In this case, the memory must be capable of functioning in manual mode.
Having unscrewed the plugs, we put the battery on recharge, and when it reaches enough capacity, the electrolyte begins to boil. If this process is observed in all six sections, it can be stated that there is no short circuit. If there are no bubbles in one of the cans, it means that it is not working, that is, it is closed.
This check should only be carried out in a ventilated area - when the electrolyte boils, flammable vapors of hydrogen and oxygen are released, which can pose a fire hazard.
You can also check the battery for a short circuit in a faster way - by closing the contacts in each section for a few seconds. In a faulty jar, you can observe the active release of gas bubbles.
Finally, a sign of a closed battery can be considered a change in the color of the liquid - from transparent it becomes dark. A drop in electrolyte levels may also indicate a similar problem.
What causes sulfation and how to avoid it?
First, let's list the reasons for sulfation:
- Operating the battery at temperatures above +40 degrees Celsius;
- Constant short-term recharge or single long-term;
- Storing the battery in a discharged state;
- Supplying a serious load when the battery is severely discharged;
- Low temperature load.
How to understand that plate sulfation has occurred:
- Decrease in battery capacity;
- Strong boiling, gas formation;
- Increased potential at the electrodes;
- The plates are warped and are heating up.
Preventing plate sulfation and other battery malfunctions:
- Once a quarter you should check the electrolyte level in the jars and measure the density;
- When charging the battery, use a current of no more than 1/10 of the battery’s rated capacity;
- In the cold season, the electrolyte density can be increased to 1.4 g/cm. cubic;
- In severe frosts, it is not recommended to leave the car outdoors. This may cause the electrolyte to freeze and damage the plates.
Many car owners are interested in whether they need to charge a new car battery after purchase? By following the link you can read an article on this topic.
Reasons for shorting battery sections
No one is immune from troubles, but drivers who do not neglect manufacturers’ recommendations experience them much less often. Moreover, proper operation of the battery does not require any serious waste of resources - you only need to check the electrolyte level several times a year and measure its capacity.
Let's look at the main reasons why the battery shorts out:
- Sometimes there are manufacturing defects, but such cases are covered under warranty;
- often short-circuiting of plates within a section occurs as a result of mechanical impacts (for example, strong impacts) on the battery body;
- The most likely reason for the short circuit of the cans is considered to be a violation of the charging mode - undercharging, if the load on the generator is too high, or overcharging (common with a faulty relay regulator).
All that is required from the car owner to prevent short-circuiting of the battery plates is to keep it clean (this will reduce the amount of current leakage), not leave it inoperative for a long time, and try to prevent the battery from being exposed to severe frost for a long time. Often, because of one inoperative battery, the entire battery has to be scrapped, so the above precautions will not be superfluous.
Accelerated battery recovery option
For those for whom time is too valuable, the following battery recovery option is suitable:
- Fully charge the battery;
- Drain the filler;
- Rinse the internal cavity of the battery with distilled water;
- Pour a solution of Trilon B (2%) and ammonia (5%) into the battery;
- After an hour, drain the mixture, rinse the “insides” again with distilled water;
- Pour in fresh acid solution;
- Fully charge the device.
It is possible that the solution with Trilon B and ammonia will have to be poured an additional 1-2 times. The process is considered complete if no gas is released when the mixture enters the device.
What batteries can be restored?
Before you restore your car battery, you should know that this procedure does not help in all cases. It is possible to “revive” a car battery only in the situations listed below:
- when during operation its plates are not destroyed by high current loads and there are no closed cans in the battery;
- if they have not received irreparable mechanical damage;
- when the battery has become unusable due to natural sulfation.
Restoring a battery is considered an almost impossible task if its plates are severely damaged.
Another option for a critical battery malfunction is when the cans are shorted, swollen, or other mechanical damage is detected.
In all other cases, restoration of the product is possible, but only subject to strict adherence to time-tested technologies.
Restoring battery terminals
When the battery is used continuously, a thick white coating appears on the terminals. The reason is an oxidation process that corrodes the metal.
What not to do when battery terminals are oxidized:
- use Vaseline for cleaning, as it impairs conductivity;
- treat parts with gasoline, as it can self-ignite, and melt the plastic case.
Restoring the terminals is done using soda. The process goes like this:
- For a soda solution you will need 1 tbsp. soda and a glass of warm water.
- The engine of the car is turned off, the nut is loosened and the terminals are removed.
- The battery is removed and checked for cracks or leaks.
- In case of wire defects, the problems are eliminated.
- For safety, it is better to wear rubber gloves.
- Using an old toothbrush soaked in the solution, carefully clean the terminals.
- To remove corrosion from battery cables, place them in hot water.
- The battery is rinsed with cold water and wiped dry.
- The terminals are treated with protective lubricant and reconnected.
IMPORTANT! If the coating on the terminals is too thick, use sandpaper and a sharp knife for repairs. However, actions must be careful. Before checking the operation of the battery, you should make sure that all parts are securely fastened.
When the battery is short-circuited, the terminal melts and loses its previous shape. To restore a part at home, use improvised means:
- Traces of melting are cleaned from the terminals.
- The remains are cleaned and tinned with a soldering iron.
- A tin mold is suitable for the new terminal.
- Gradually fill the mold with the prepared solder and mix lightly.
- A cutter is made from a water pipe or a brace.
- The workpiece is processed with a milling cutter.
- Using sandpaper and a file, shape the terminal.
IMPORTANT! In car services it is possible to restore the old terminal. To prevent battery damage, it is better to make a new one. There is no difference between the positive and negative terminals.
If the battery is not completely discharged: how to start a car with a discharged battery
It happens that the battery was almost able to start the car, but after some small actions it stopped responding to subsequent attempts. This most likely indicates that the battery is not completely discharged. This outcome is the most favorable, since in this case there is an opportunity to correct the situation and start the car with such a battery.
Charger or spare battery
You can recharge the battery quite simply and quickly if you have a battery charger with you. Or, for example, a spare battery. The main thing is to set aside 20-30 minutes for charging and the car will start again. You can also simply change the battery or recharge a failed one from a spare one.
Advice! To be always prepared for technical imperfections, it is recommended to always have a battery charger in the trunk. It’s even better if you have a spare battery with you. It doesn't have to be new; it can be a reconditioned car battery. It won't last long, but the time gained will be enough.
In addition, portable boosters using lithium-ion batteries have become widespread. The only drawback of the latter is the specificity of lithium-ion batteries. They should not be left in a car at sub-zero temperatures outside.
It is worth understanding that recharging the battery from a portable charger every time is harmful to the condition of the battery. It is also better to pay attention to how long the battery holds a charge after this. If it lasts at least 3-7 days, then everything is in order and the battery will last for some time. Otherwise, it is worth starting to carry out a full diagnosis to find out the reason why the battery is quickly discharged, or what is preventing it from producing current.
Read more in the article: “The car battery discharges quickly: reasons and their solutions”
Plant with pusher
A simple and often used way to start a car with a dead battery is to push start it. This method can only be used in cars with a manual transmission.
You need to push the car and try to start it while driving. It is better to do this on level ground, since here the car will not be able to roll down in case of failure.
In the case of an automatic transmission, this method cannot be used on modern cars.
Read also:
Gel or AGM?- How the coming heat can finish off a still fresh battery
- The latest battery forming technology has been introduced at the Kursk Battery Plant
- The Chinese have developed power capacitors that could change the idea of electric vehicles
If you installed a battery with a larger capacity than the standard one, did the starter burn out?- Lithium-ion batteries will become safer thanks to a popular ingredient in skin creams
Tags:: SHUBA, car battery, battery charging, battery protection, electrolyte concentration, battery overheating, battery self-discharge, battery life, plate sulfation, electrolyte temperature, battery thermal cover, battery insulation, battery cover
Popular battery types
Lead-acid types of batteries are now the most common in vehicles.
Despite the use of an electrolyte (a solution of sulfuric acid in distillate), which makes it possible to carry out chemical reactions accompanied by the release of an electrical discharge, such batteries are considered safe, but only conditionally.
The batteries have sealed housings that prevent electrolyte leakage to the maximum. But still they are only conditionally safe.
And all because the leakage of an acid solution as a result of damage to the housing (crack, etc.) is not the most dangerous; the greatest danger is the possibility of an explosion with scattering of housing particles and electrolyte drops.
For many motorists, it may seem that the battery cannot explode, simply because there is nothing in its design related to explosive or flammable substances, but the fact remains that such cases do occur.
As noted, the operation of a battery is based on chemical reactions, which can cause the release of explosive substances.
And strangely enough, the appearance of such components is ensured by the presence of distilled water.
Please note that a battery explosion is only possible when certain factors coincide. But first, a little theory.
Electrolyte evaporation
| When the positive plate of the battery is discharged, oxygen is formed from the water. Under normal float charge conditions, oxygen recombines with hydrogen on the negative plate of the battery, restoring the original amount of water in the electrolyte. But oxygen diffusion in the separator is difficult, so the recombination process cannot be 100% effective. Reducing the proportion of water changes the charging characteristics of the battery and, at a certain threshold, makes charging completely impossible. Factors that increase the risk of “battery drying out”:
|
Electrolyte evaporation is an irreversible phenomenon for gel and AGM batteries. The main reason for drying out, especially for AGM, is “overcharging” the batteries.
Active battery equalization
You can smooth out differences in battery parameters using a special device called a battery charge balancer or imbalance leveler.
| The unbalance leveler SBB2-12-A equalizes the state of charge of two 12V batteries connected in series or several such parallel chains. The operating principle is based on the active redistribution of the charge of battery cells, in which almost the same voltages are established on adjacent batteries. level provides charge redistribution between batteries even in the absence of charger current. |
IMPORTANT! The use of charge balancers reduces the risk of destructive processes, but cannot fix an already seriously damaged battery.
Physically, the battery charge equalization device is a compact electronic module connected to each pair of series-connected elements:
- for a 24V battery, one charge balancer per chain is required
- a 48V battery requires three charge balancers per string (diagram 2).
The SBB is powered from the battery itself or from a charge source. SBB's own power consumption is small and comparable to losses due to self-discharge.
The efficiency of the SBB2-12-A level is fundamentally higher than that of other charge balancers, the operation of which is based either on shunting excess charging power (so-called passive balancers, creating direct energy losses), or on selective recharging of elements (equalization occurs only during charge). The maximum equalization current of SBB2-12-A is 5A, which exceeds the capabilities of all alternative devices on the market.
Results
As you can see, it is possible to prevent sulfation of battery plates and this is not difficult to do: just follow the simple recommendations given above. By following them, you can significantly extend the life of your energy source, count on its reliable operation in any situation, and also save your family budget, which is also important.
If, after all, trouble strikes, then taking timely measures will allow you to avoid serious consequences and revive the battery. Having information about the first manifestations of the process that has begun and not forgetting about periodic visual inspection of the engine compartment, you can manage to get rid of the problem at the initial stage.
If you strictly follow the rules of car operation and maintenance procedures, the appearance of even signs of battery sulfation can be avoided.
Corrosion and shedding of the active substance
| During corrosion, pure lead of the plate grid, interacting with water, is oxidized into lead oxide. Lead oxide conducts electric current worse to the active substance of the plate lubricant, increases internal resistance and reduces the battery's resistance to high discharge currents. On the positive plates, corrosion weakens the adhesion of the grid to the active substance. In addition, the active substance of the positive plate itself gradually loses strength. With each cycle of spreading, the layer of the plate changes state from a bulk mass of microcrystals of lead oxide to a hard crystalline structure of lead sulfate. Alternating compression and expansion reduces the physical strength of the spread layer, which, combined with a weakening of adhesion, leads to sliding and shedding of the active substance to the bottom of the battery. Corrosion and accumulation of detached active substance can lead to deformation of the battery plates and, in the worst case scenario, to short circuit. |
Factors that increase the risk of corrosion and shedding of the active mass:
- charge too high voltage;
- charging with insufficient current - that is, staying under high voltage for a long time during the filling phase;
- staying in the absorption phase for too long (“overcharge”);
- charging the battery with too much current;
- accelerated battery discharge with too much current.
Shedding (sliding) of the active mass of the electrolyte is an irreversible phenomenon. The most dangerous consequence of sliding of the active mass is the shorting of the plates.