Many drivers, leaving the car for a while, then discover that it does not want to start. At this time, the starter may not show any signs of life at all. Most likely the reason is the battery, which has been discharged in a few days. Charging in such a situation does not always help. The root of the problem is a decrease in electrolyte density. A car service center will help you return it to normal, but many people prefer to act on their own. How to increase the electrolyte density in a battery with your own hands and why its level changes is in the article. In general, this is a common occurrence, and a battery that lasts 3–5 years is considered a consumable item.
Equalizing the density of the electrolyte will help extend the life of the battery.
What is the density of electrolyte in a battery?
A lead-acid battery contains containers called "cells". They contain sulfuric acid, into which lead plates are immersed. If the electrolyte boils away, its chemical composition changes, or sulfation occurs (damage to the plates), the battery may fail. In non-critical situations, there are ways to restore the power source at home.
The electrolyte itself is a liquid that serves as a catalyst for the electrochemical process. Without it, a battery is nothing more than plastic filled with lead. The electrolyte consists of approximately 65% distilled water and 35% sulfuric acid. One of the indicators of this “chemistry” is density, which can either fall or rise, depending on the charge of the battery.
How a car battery works
The main function of a car battery, in fact, is the accumulation and storage of electrical energy, which occurs through a chemical reaction through the interaction of the electrolyte and lead plates. It is thanks to these processes that you get a full-fledged autonomous power source. Not only the success of starting the engine, but also the operation of other autonomous vehicle systems depends on the condition of your battery.
Each battery has its own specific current for cold starting the engine. It can be different, so the battery is selected individually for each engine, for example, for a diesel engine with a volume of 2500, the minimum starting current should be at least 600-650 amperes, but it is better to use 750 A. And the amount of time during which the battery can be under load turning the starter is called battery capacity. This indicator is measured in A/h.
But still, the principle of operation and malfunctions of all batteries are the same. The principle of operation is simple: lead plates are sealed in a plastic case, and the space between them is filled with a solution of sulfuric acid of a strictly defined density. The acid concentration is directly related to the density; the more acid there is, the higher the density. The second component is distilled water (completely purified from foreign impurities).
What factors affect density
Before figuring out how to increase the density in a battery, it is necessary to identify why it may fall. The reasons are as follows:
- discharge. Less charge means lower density of sulfuric acid. During charging, the parameter gradually increases. When a battery has been in use for a long time, part of its energy capacity is lost, which is accompanied by a drop in electrolyte concentration;
- the battery life is coming to an end, or storage rules have been violated (if you leave the car in cold conditions for the winter, this will damage the battery);
- Some of the electrolyte has boiled away due to overcharging. When the charger provides increased voltage, the liquid turns into a gas and is removed through the vents on the battery case;
- The car owner often adds water to maintain the electrolyte level. When doing this, you need to get a device for measuring density, since the acid boils away along with the water. The result is low concentration.
The density value determines how efficiently the power supply will hold a charge. For this reason, it is recommended to periodically measure the parameter, which will help identify the problem in time. If ignored, the lead plates will deteriorate prematurely.
When the density indicator is low, the car simply will not start. In such cases, it is necessary to increase it, thereby restoring the functionality of the battery.
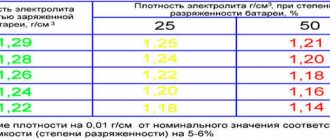
Electrolyte density value for battery.
Why are low and high electrolyte concentrations dangerous?
Due to the increased concentration, the battery will fail faster. Acid is destructive to metal plates - “chemistry” can even ruin steel.
Low concentration leads to:
Sulfation of the battery plate.
- sulfation. This is the name of the process when a whitish coating appears on lead plates - lead sulfate deposits. With them, the battery takes a charge worse, or even refuses to charge at all;
- increasing the freezing threshold. When the density is 1.28 g/cm3, the liquid freezes only at a temperature of -58°. If it is lower, it will freeze even at -5°. Ice damages the plates, increasing the risk of a short circuit, after which it is no longer possible to “reanimate” the battery;
- problems with starting the engine, especially in winter.
Preventive measures and tips for battery operation
During operation of a car battery, it is necessary to remove contaminants from the surface of the case. The settling electrolyte vapors form tracks between the poles, which leads to accelerated discharge. Older cars do not have an electronic charging control system, so it is necessary to periodically check the voltage in the on-board network. When using a CAN or LIN digital bus, the control unit automatically maintains the battery in a charged state.
If the car is operated in regions with minimum temperatures below -50°C, then the electrolyte density must be increased to 1.29 g/cm³ (when fully charged). Additional sulfuric acid allows you to lower the crystallization point of the solution. If the car is rarely used in winter or the driver makes frequent trips over short distances, then the battery should be periodically charged from an external device.
How to check electrolyte density
The procedure is simple, you can do it yourself. It is recommended to carry it out at room temperature. Before you begin, prepare the following:
- personal protective equipment (gloves, goggles). If electrolyte gets on exposed skin, it can cause a burn. Chemical vapors are also dangerous, so work is carried out in a well-ventilated area;
- densimeter. This is the name of a device for measuring density. It looks like a glass tube with a bulb that has a built-in hydrometer.
How to measure density correctly:
- Remove the power source from under the hood, remove the protective cover, if there is one, and unscrew the plugs.
- First, the electrolyte level is assessed. In the case of lead-acid batteries, it should cover the plates by 1.5 cm.
- Fully charge the battery. After charging, allow it to “rest” for 5–6 hours, and then measure the density. If the level is normal, immerse the device in jars and pump out some liquid. The hydrometer should float freely without touching the walls.
- They look at the indicators and compare them with normal values (the norm depends on the region, since climatic conditions are different everywhere).
In this way, you can only check the battery being serviced. If the battery is maintenance-free, there is an indicator, the color of which indicates the level of electrolyte density.
Electrolyte density indicator.
Preparing the Battery
When a car enthusiast knows why the density of the electrolyte in the battery has dropped, then there is no need to immediately raise it. It is necessary to carry out some preparatory activities. To do this, you must ensure that several conditions are met:
- The battery is charged.
- The temperature of the solution in the batteries is from 20 to 25 degrees.
- The electrolyte level in each jar is normal.
- The battery has no mechanical damage.
We recommend: Norm of current leakage from a car battery
If the battery is discharged, its capacity should first be restored. Then the density of the solution must be measured, since the acid concentration may be below normal. A situation is possible in which this solution parameter after recharging in batteries is different. It should be remembered that the permissible difference in this indicator is a maximum of 0.01 kg/cm3. To equalize the density values in the banks, it is necessary to carry out corrective recharging. This is done as follows:
- The current strength is reduced by 2-3 times compared to the nominal value.
- The battery charges within 1-2 hours.
If this method does not help solve the problem, you will have to take a more serious measure by adding a correction electrolyte solution. It should be noted here that it can only be used as a last resort.
An electrolyte whose density is 1.4 kg/cm3 is called a correcting electrolyte.
First, the car owner should check the battery and find out why the concentration of the solution disappears. If this happened due to boiling water, then the correction electrolyte cannot be added. It is used only in two cases:
- A liquid leak has been detected from the batteries.
- A lot of distilled water was poured into the jars, which caused a decrease in the density of the solution.
Enhancement Methods
For the engine to start properly, the power source must be charged, and experienced drivers ensure this. But situations are different. There are several ways to increase density, which vary in complexity.
Correction electrolyte
Increasing the density in this way is carried out in stages, and in order to achieve the desired result, consistency is required.
Before starting work, you should prepare: a hydrometer, glass containers, a bulb, protective equipment, water and correction electrolyte. The battery must be warmed up - just leave it in a warm room for several hours.
Procedure:
- The power supply is fully charged, which takes at least 8 hours.
- Chemical indicators are measured. It should be from 1.25 to 1.27 g/cm3.
- If it is lower, the electrolyte is partially drained from the cans.
- Next, you need to add a correction electrolyte to them (half as much as was pumped out).
- Add water to cover the lead plates.
- The battery is charged for 30-60 minutes, and then they wait a couple of hours for the liquid to mix.
- The indicators are measured again.
If the density does not rise, repeat the steps.
Many people do not buy, but make the corrective electrolyte themselves. In this case, you must first add water, and only then the acid solution.
Preparation of electrolyte from water and acid.
Alignment
This method is suitable if the plates are free of defects or have slight plaque. Before proceeding, charge the battery with a low current. After 12 hours, charge again, setting the voltage to 14.6 - 14.8 V.
The method is often used in winter, when the electrolyte concentration drops. They restore with weak currents, but charging can take up to three days.
How it works: the acid solution begins to “boil”, which is accompanied by bubbles on the surface, and when the excess liquid evaporates, the concentration of the electrolyte increases. Since it will eventually become less, you will have to add a new solution, and only then measure the density and look at the hydrometer readings.
The method, although long, is effective, especially when there is no other way to restore the battery.
How to boost using a charger
If the acid concentration has dropped over the winter, it can be restored by applying a weak current. Charging takes at least 3 days, it is considered effective if it is impossible to restore the battery using other methods. The contents of a battery that has reached full power begins to boil when charging. A sign of water evaporation is the formation of small bubbles on the surface.
Excess liquid will evaporate, the acid concentration will increase. The total fill level will become low, so you will have to add a ready-made battery solution. After completing the procedure, use a hydrometer. If the device readings are too low, repeat charging and adding electrolyte.